Running Head: Assessment of Obstructive Sleep Apnea
Funding Support: Dr. Feemster received support during the period of this work from the National Institutes of Health (K23 HL111116). Dr. Donovan received support during the period of this work from VA Health Services Research and Development CDA 18-187. The views expressed in this article are those of the authors and do not necessarily represent the views of the United States Department of Veterans Affairs. Dr. Keller received support from T32 5T32HL007287-39. This work was performed at the University of Washington.
Date of Acceptance: December 5, 2023 │ Published Online Date: December 13, 2023
Abbreviations: BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; EMR=electronic medical record; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Lung Disease; ICD-9=International Classification of Diseases, Ninth Revision; OR=odds ratio; OSA=obstructive sleep apnea; PAP=positive airway pressure; PCP=primary care provider
Citation: Donovan LM, Keller TL, Stewart NH, et al. Assessment of obstructive sleep apnea among patients with chronic obstructive pulmonary disease in primary care. Chronic Obstr Pulm Dis. 2024; 11(2): 136-143. doi: http://doi.org/10.15326/jcopdf.2023.0438
Online Supplemental Material: Read Online Supplemental Material (212KB)
Introduction
Obstructive sleep apnea (OSA) is a persistent source of concern in the management of chronic obstructive pulmonary disease (COPD).1 Patients with COPD and comorbid OSA have a greater burden of sleep-related hypoxemia and sleep fragmentation than either condition alone.2,3 Among patients with COPD, observational cohorts link OSA to a greater risk for COPD exacerbations, hospitalizations, and mortality.4-6 Observational studies also suggest that these risks may be mitigated with positive airway pressure (PAP).4,5,7-9 The prevalence of moderate to severe OSA among patients with COPD ranges from 10%–30%, however, most patients are undiagnosed and untreated.2,10 Although we lack high-quality evidence to support screening,11 many providers and some professional society statements advocate for the regular assessment of general sleep symptoms and OSA-specific sleep symptoms among patients with COPD.12,13
A directed clinical history is essential to ensure appropriate diagnosis and management of OSA.14 Among those with undiagnosed disease, the identification of OSA symptoms including snoring, witnessed apneas, and daytime somnolence is often necessary to prompt diagnostic testing.15 Similarly, providers need to assess symptoms and treatment use among those with established OSA to ensure the adequacy of current management.16 However, such assessments require staff time and we need to acknowledge competing demands, particularly in primary care practices where the vast majority of patients with COPD receive care.17-19
Faced with competing demands and a lack of high-quality evidence, it is unclear how often OSA assessment occurs among patients with COPD. If we are to fully understand barriers and opportunities to OSA treatment, we need to learn more about this initial step. We sought to address this knowledge gap using comprehensive chart reviews of patients with a clinical diagnosis of COPD in 2 large academic primary care practices. We also aimed to assess patient and provider characteristics associated with OSA assessment.
Methods
Our cross-sectional sample includes patients with clinically diagnosed COPD who were seen from June 2011 to June 2013 at 2 primary care clinics affiliated with the University of Washington Together, these clinics serve over 11,000 patients per year at both a tertiary referral center and a public safety net hospital. We included patients with an outpatient or inpatient discharge diagnosis of COPD based on the International Classification of Diseases Ninth Revision (ICD-9) codes20 of 491.X, 492.X, 493.2, or 496.X. To ensure inclusion of patients with adequate opportunity to have OSA assessed, we only included patients with at least 2 outpatient primary care visits within a 1-year span, and defined index dates as the date on which patients met cohort entry criteria. We identified patients using administrative data from the University of Washington electronic health record. The University of Washington Institutional Review Board approved this study (UW 41666 EA).
Our main outcome was primary care assessment of OSA based on the presence of documentation regarding: (1) the presence or absence of OSA symptoms, (2) PAP treatment, or (3) referral to sleep medicine in the year prior to index. As these patients are frequently comanaged with pulmonary for COPD care, we also abstracted any available pulmonary specialist notes.
Symptoms: Our chart reviews documented whether providers mentioned the generic presence or absence of “OSA symptoms” or the specific OSA symptoms of snoring, daytime somnolence, witnessed apneas, and/or gasping/choking arousals.
PAP Treatment: We recorded whether providers documented a patient’s use or nonuse of PAP.
Sleep Referrals: We assessed whether patients were referred to sleep medicine, had a sleep study performed, and/or were seen in sleep clinic by a sleep specialist physician or sleep-focused respiratory therapist. We considered including diagnostic codes for OSA in our composite outcome, but deferred as these codes have poor validity in current clinical practice.21 In a sensitivity analysis, we expanded our composite outcome to include the presence of an ICD-9 billing code for OSA within the electronic medical record (EMR) during our 1-year time period of interest. The chart reviews included in our analysis were conducted by 2individuals: one pulmonary physician and one research assistant. These individuals conducted duplicate chart abstractions22 for approximately 20% of the reviews and discrepancies were noted to be less than 5%.
To better understand associations at the patient and provider level, we included patient variables related to OSA risk. We also included characteristics of patients and their primary care providers (PCPs) that we hypothesized to influence patient-provider communication and the comprehensive assessment of symptoms.
Patients: We included demographics (age, sex, marital status, insurance, race) and body mass index (BMI) (categorized as <25, 25–30, 30+ kg/m2, or unknown) recorded nearest to the index date. We included: (1) comorbidities; (2) outpatient health care utilization (total number of PCP and pulmonary visits); (3) outpatient primary care fragmentation (number of unique PCPs seen, proportion of primary care visits with most frequent PCP) in the year prior to index; (4) inpatient COPD exacerbations in the 2 years prior to index; and (5) pulmonary function testing recorded in the EMR in the 5 years prior to index Finally, we recorded whether sleep studies diagnosing OSA were present in the EMR. To avoid concurrence with our outcome assessment period, we only included prior sleep studies documented from 10 years prior to index up until 1 year prior to index. (All sleep studies recorded in the year prior to index were included in our composite outcome of OSA assessment.)
PCPs: We included the characteristics of gender, years since completing terminal clinical degree, and whether they were an attending physician, resident physician, or advanced practice provider at time of index.
Statistical Analyses
Our primary analytic model of interest was a mixed effects logistic regression model including both patient and primary provider characteristics. This modeling strategy allowed us to simultaneously account for contributors both at the patient and provider level. We report effect estimates for each covariate from univariate and multivariate models. As sensitivity analyses, we performed separate logistic regression models with: (1) a composite outcome that also includes ICD-9 diagnostic codes for OSA, and (2) a sample restricted to patients with COPD confirmed by spirometry (forced expiratory volume in 1 second [FEV1] to forced vital capacity ratio <0.70) with severity of ventilatory impairment by FEV1 included as a covariate. We conducted all analyses in STATA (StataCorp, Version 16, College Station, Texas).
Results
We identified 641 patients with COPD seen in our primary care clinics. In Table 1, we summarize the characteristics of our sample and their primary care providers. Our sample’s characteristics overlapped with a number of OSA risk factors including older age (mean age 64 years), majority male sex (60%), and overweight and obesity (high prevalence: combined 63%). Our sample also included a high prevalence of several comorbidities known to predispose patients to OSA including type 2 diabetes, hypertension, and coronary artery disease. At baseline, prior sleep studies available within the local EMR confirmed the presence of OSA among 11% of patients.
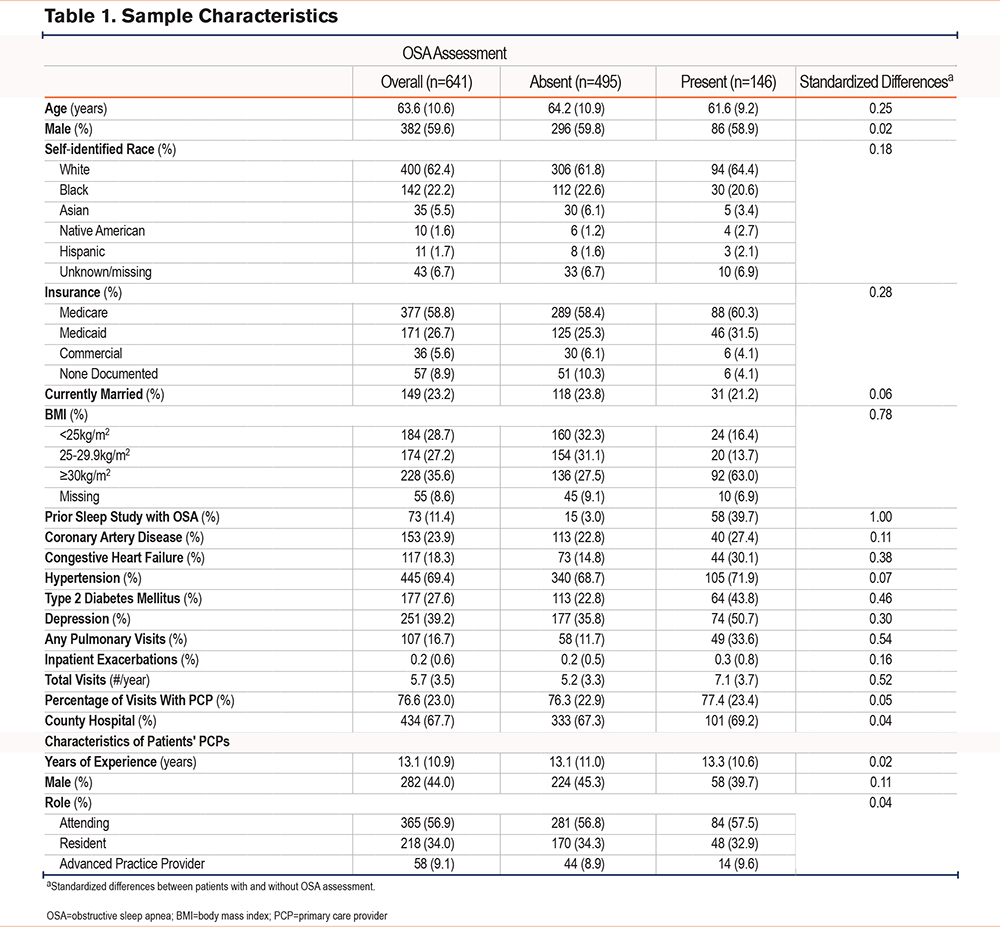
Overall, 146 patients (23%) met our combined outcome of OSA assessment, demonstrated by at least 1 of the following: documented assessment of OSA symptoms (n=134), documented assessment of PAP usage (n=107), or referral to sleep medicine (n=81). We include the characteristics of individuals who did and did not meet our primary outcome in Table 1. We further explored the assessment of OSA symptoms. Among the 134 patients with documented assessment of OSA symptoms, providers only documented general symptoms of OSA in 77 (58%). Among these 77 individuals, providers’ documentation consisted only of generic phrases such as “OSA stable” or “denies OSA symptoms.” In the remaining 57 patients, providers documented the assessment of specific symptoms. The most commonly assessed symptom was daytime somnolence (n=44), followed by snoring and witnessed apneas (both n=21).
In mixed effects logistic regression models, patients with a higher BMI, history of type 2 diabetes, major depression, pulmonary subspecialist visits, and a greater number of total visits were more likely to have OSA assessed. Patients self-identifying as Black or African American were less likely to have OSA assessed relative to those identifying as White. We observed the strongest association with prior sleep studies confirming the presence of OSA. We did not observe significant associations with characteristics of primary care providers (Table 2).
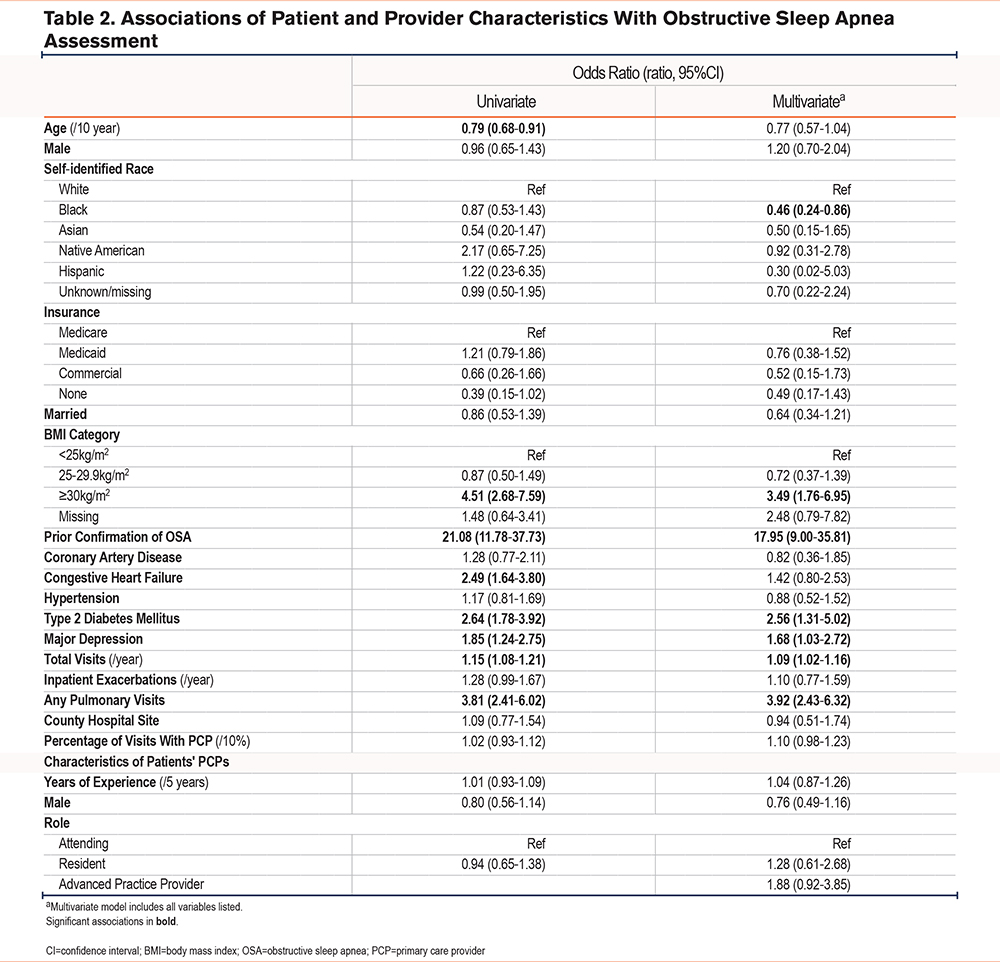
Finally, we conducted several sensitivity analyses. First, we added OSA diagnostic codes to our composite outcome, classifying an additional 16 patients as having OSA assessed. In a second sensitivity analysis, we included severity of airflow obstruction, restricting our sample to the 293 patients with COPD confirmed by spirometry with available FEV1 values. In both sensitivity analyses, the point estimates and direction of associations tracked with those observed in the primary analytic approach. Relative to patients with mild COPD, we observed no differences in the assessment of OSA among patients with more severe reductions in FEV1 (See Supplemental Tables 1 and 2 in the online supplement).
Discussion
Over the course of a year, we found that roughly a quarter of patients with clinically diagnosed COPD had documented assessment of OSA. Despite greater rationale around OSA assessment in the COPD population, our findings align with those of the general primary care population where approximately one third of patients have sleep symptoms assessed.23 Our analyses also identified certain characteristics that are associated with OSA assessment, shedding light on potential mechanisms for underdiagnosis and disparities in sleep care.
Perhaps not surprisingly, the strongest predictor of OSA assessment was the presence of a past sleep study in the medical record confirming OSA. There are likely multiple mechanisms for this finding. For one, our observations might simply reflect ongoing patterns of behavior for patients and providers. Patients with known OSA may be more likely to discuss their ongoing OSA management with providers. Additionally, providers who previously asked patients about their OSA symptoms, and pursued diagnostic testing, may be more likely to continue to ask said patients about these symptoms.23 Finally, the presence of OSA documented within the medical record may prompt providers to discuss sleep symptoms during visits, as is seen in other health conditions.24
Our findings also highlight racial disparities in OSA assessment. Black adults are known to have a higher prevalence of undiagnosed and undertreated OSA relative to White peers.25-27 This disparity has been attributed to systemic inequities in access to care and additional social and geographic barriers that prevent completion of sleep referrals.27 Our findings illustrate another step at which disparities arise. Even after accounting for visit completion, insurance status, and comorbidities, we found Black patients had roughly half the odds of OSA assessment relative to White patients. Our finding suggests that efforts to address disparities in sleep care for high-risk populations like COPD should also consider ways to improve consistency of OSA symptom assessment across racial and ethnic groups.
In order to contextualize our observed rates of OSA assessment, it is important to consider the marked time limitations for primary care visits, and the multiple competing demands within these appointments.17,18 While many factors play into what is and is not discussed during a primary care visit, we need to recognize the relatively low strength of evidence in support of assessing OSA among patients with COPD. Although existing observational evidence may be compelling for the sleep community, it does not meet the highest standards of evidence relative to other health maintenance activities that primary care is tasked with completing (e.g., smoking cessation).28-30 If we are to implement OSA assessment among patients with COPD more broadly, we need stronger evidence around the benefits of identifying and treating OSA among patients with COPD. At least one ongoing randomized trial promises to deliver high-quality evidence, and we look forward to the results (Clinical Trials ID NCT04179981). In addition to improving the strength of our evidence base, we should consider ways to engage with patients that do not rely on primary care provider time or resources. For instance, streamlined collection of patient symptoms outside of primary care encounters.31,32
Our work has a number of strengths including the presence of in-depth chart reviews performed longitudinally in a large sample of patients with COPD. As a result, our analyses allow a comprehensive view into the documented assessment of OSA. Despite these strengths, there are weaknesses in our approach that merit discussion and contextualization. First, it is important to note that we are only able to measure the documentation of OSA assessment within our medical record. Providers may have discussed OSA and not documented these discussions, particularly when symptoms of OSA were absent or treatment for OSA was well tolerated. Moreover, our approach only considered documentation of OSA assessment, and not general sleep symptoms (e.g., insomnia symptoms). Therefore, the true prevalence of OSA assessment and assessment of general sleep symptoms may be higher than we report. Second, while our inclusive approach to sampling offers a generalizable view of usual care practices, it did not allow for systematic screening. Therefore, we do not know the true prevalence of OSA in our sample and cannot say with certainty the number of patients whose OSA was undiagnosed or not reassessed. Nevertheless, longstanding recommendations suggest that all patients receive assessment of OSA, and recent evaluations10 suggest the prevalence of OSA in the COPD population to be up to 65%. Finally, it is important to acknowledge the timeframe of our cohort, 2011–2013. While many drivers of OSA assessment remain (e.g., lack of time in primary care, limited evidence base around the role of OSA screening in COPD),33,34 it is possible that OSA assessment has either increased or decreased since 2013. For instance, convenient and scalable home sleep apnea tests have extended the reach of OSA diagnostic services and have contributed to increased OSA diagnoses and awareness.15,35,36 However, current American Academy of Sleep Medicine guidelines specifically advise against home sleep apnea test use among patients with COPD.15 Additionally, while the recent Global Initiative for Chronic Obstructive Lung Disease13 statements advocate for regular assessment of sleep symptoms generally, these recommendations are not focused on OSA like the 2004 American Thoracic Society/European Respiratory Society guidelines. Finally, since 2013, several trials have failed to identify a benefit for continuous positive airway pressure in secondary prevention of cardiovascular disease.37-39 It is possible that these results could have dampened enthusiasm in primary care around the detection of OSA in other populations like COPD. Due to these and other potential drivers, future work should reassess our findings in more recent cohorts.
Overall, we found that providers assess OSA among roughly a quarter of patients with COPD. As a field, we need higher quality evidence around the impacts of identifying and addressing OSA in COPD. Lacking high quality evidence, one can argue that primary care providers who do not ask about OSA are simply making prudent choices around time allocation. We need to understand whether the assessment of OSA improves meaningful outcomes in COPD, and, if effective, how such assessment can be accomplished in a sustainable and equitable manner.
Acknowledgements
Author contributions: LMD is the guarantor of this manuscript and takes responsibility for the content, including the data and analysis. All authors met authorship requirements. LCF, TLK, and DHA were responsible for study design and collection of data. Analysis of data was provided by LMD, LCF, and TLK. All authors interpreted the data, assisted in preparing the manuscript and approved of the version of the manuscript submitted for publication.
Declaration of Interests
Dr. Au reports personal fees from Boehringer Ingelheim for service on an advisory board and personal fees from the Annals of the American Thoracic Society for service as a deputy editor. Dr. Feemster reports personal fees from the Annals of the American Thoracic Society for service as an associate editor. Dr. Feemster receives consulting fees from the National Committee for Quality Assurance for curriculum development and the Society for Hospital Medicine and the American Thoracic Society to serve on expert panels. Dr. Donovan reports a speaking honorarium from the American Academy of Sleep Medicine. All other authors have no declarations.