Running Head: COPD Patient-Reported Outcomes With Revefenacin
Funding Support: Support for this exploratory post hoc analysis and development of this manuscript was funded by Theravance Biopharma U.S., Inc, San Francisco, California. The funders had no role in the study design, data analysis, results, interpretation, or writing of the manuscript.
Date of Acceptance: January 4, 2024 | Publication Online Date: January 19, 2024
Abbreviations: −A/−D=neither anxiety nor depression; +A/+D=both anxiety and depression; A=anxiety only; AE=adverse event; BMI=body mass index; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; D=depression only; FEV1=forced expiratory volume in 1 second; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; LS=least squares; MCID=minimum clinically important difference; MedDRA= Medical Dictionary for Regulatory Activities; PRO=patient-reported outcome; QoL=quality of life; SGRQ=St George’s Respiratory Questionnaire; TEAE=treatment-emergent adverse event
Citation: Yohannes AM, Iyer AS, Clay C, et al. Post hoc analysis of lung function improvement and patient-reported outcomes with revefenacin in adults with moderate-to-very severe COPD and comorbid anxiety or depression. Chronic Obstr Pulm Dis. 2024; 11(2): 196-205. doi: http://doi.org/10.15326/jcopdf.2023.0465
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic inflammation and progressive lung function decline that affects over 392 million people worldwide.1,2 COPD is associated with impaired quality of life (QoL), reduced social interactions, high health care utilization, and caregiver burden, especially in the presence of comorbidities.3 Over 60% of patients with COPD exhibit 2 or more chronic comorbid conditions.4-6 Of these conditions, anxiety and depression are common, and the prevalence of clinically relevant anxiety and depression symptoms in patients with COPD is estimated to range from 22% to 48% for both.7-9 Underlying anxiety and/or depression symptoms are often underreported, underdiagnosed, and undertreated, and can predict severe respiratory exacerbations and severity of COPD and asthma,10-12 which can result in impaired QoL and increased health care utilization compared to patients without these symptoms.
Untreated anxiety and/or depression compounds patients’ COPD by worsening several outcomes, including increased physical disability, elevated dyspnea, early dropout from pulmonary rehabilitation programs, increased exacerbation risk, increased episodes of hospital readmissions, and poor adherence to COPD therapies.13-17 Given that sensory information from breathing activates cortical regions of the brain to create the perception of dyspnea, it is apparent that there is a bidirectional relationship between emotion and dyspnea, with heightened anxiety and/or depression perhaps leading to increases in ventilation and worsening of dyspnea.18,19 Furthermore, alleviating dyspnea through bronchodilators may help to reduce the baseline for dyspnea perception and the effect of anxiety and/or depression on elevating dyspnea symptoms. In addition, treatment of underlying psychiatric disorders in patients with COPD improves pulmonary status and other respiratory outcomes.20,21
Bronchodilators are central to the management of COPD symptoms.22 However, a recent observational study examining the level of adherence to inhaler therapy in patients with COPD in a primary care setting found that ~75% of patients were nonadherent.23 In another study the presence of dyspnea in patients with COPD was significantly associated with depression symptoms and low treatment adherence to COPD medications was correlated with depression scores and negative long-term outcomes.24 To date, factors that contribute to low adherence to inhaler therapy in patients with COPD are poorly understood. The complex multifactorial problem of nonadherence includes physical disability (e.g., dexterity problems), psychological (e.g., elevated anxiety or depression) and/or cognitive impairment, costs related to medications, and difficulty managing different inhaler devices.25,26 Together with poor inhaler adherence, individuals with comorbid anxiety and/or depression may also have altered symptom perception and may, therefore, respond differently to bronchodilators. However, this has not been well studied. Medication adherence, therefore, should be a key factor when treating the respiratory symptoms of patients with COPD who may also have comorbid anxiety and/or depression. This is exemplified among adults with asthma where major depression was associated with a decrease in bronchodilator response and a worsening of respiratory outcomes.27
Revefenacin is a once-daily nebulized, long-acting muscarinic antagonist (LAMA) approved in the United States for the maintenance treatment of COPD.28 In phase 3 clinical trials, revefenacin demonstrated statistically and clinically significant improvements in lung function and QoL versus placebo in patients with moderate-to-very severe COPD.29,30 Revefenacin was also well tolerated in these trials, with high medication adherence rates (more than 90% of patients had adherence rates of 80% or more).29 Here we examine the potential benefits of revefenacin, specifically in the management of patients with COPD who also have self-reported comorbid anxiety and/or depression, by conducting an exploratory post hoc analysis of pooled data from two 12-week phase 3 trials. Utilizing these pooled data, we evaluated lung function via trough forced expiratory volume in 1 second (FEV1) at Day 85 and COPD-specific health-related patient-reported outcomes (PROs) in patients with moderate-to-very severe COPD with comorbid anxiety and/or depression.29,30 Due to the once-daily dosing regimen, ease of administration, and previously demonstrated high adherence rates, we hypothesized that the impact of revefenacin on lung function and PROs would be consistent across the anxiety/depression subgroups of patients with COPD.
Methods
Trial Design and Patients
For this exploratory post hoc analysis, data were pooled from two 12-week, replicate, randomized, double-blind, placebo-controlled phase 3 trials (0126 [NCT02459080] and 0127 [NCT02512510]) in patients with moderate-to-very severe COPD as described previously.29 The protocols for these phase 3 trials were approved by the institutional review boards at participating sites and written consent was obtained from patients before trial procedures were initiated.
Briefly, the trials enrolled patients (aged ≥40 years) with spirometric confirmation of COPD, a post-ipratropium FEV1 to forced vital capacity ratio of 0.7, a post-ipratropium FEV1 <80% of predicted normal, and a smoking history of ≥10 pack years. Key exclusion criteria included: (1) a history of myocardial infarction or unstable angina in the previous 6 months, (2) unstable or life-threatening cardiac arrhythmia requiring intervention in the previous 3 months, (3) a New York Heart Association Classification of Heart Failure Class IV heart failure prior to trial initiation, or (4) an abnormal and clinically significant 12-lead electrocardiogram at trial entry. Patients were randomized 1:1:1 to receive revefenacin 88 µg, revefenacin 175 µg, or placebo once daily in the morning administered via the PARI LC Sprint® jet nebulizer (Pari Respiratory Equipment, Inc., Starnberg, Germany) for 12 weeks. Only patients who received revefenacin 175 µg (the U.S. Food and Drug Administration−approved dose) or the placebo were included in this exploratory analysis (trial overview in Figure 1). Concomitant long-acting beta2-agonist (LABA)–containing therapy (with or without inhaled corticosteroids) was used in 37% of the trial population.
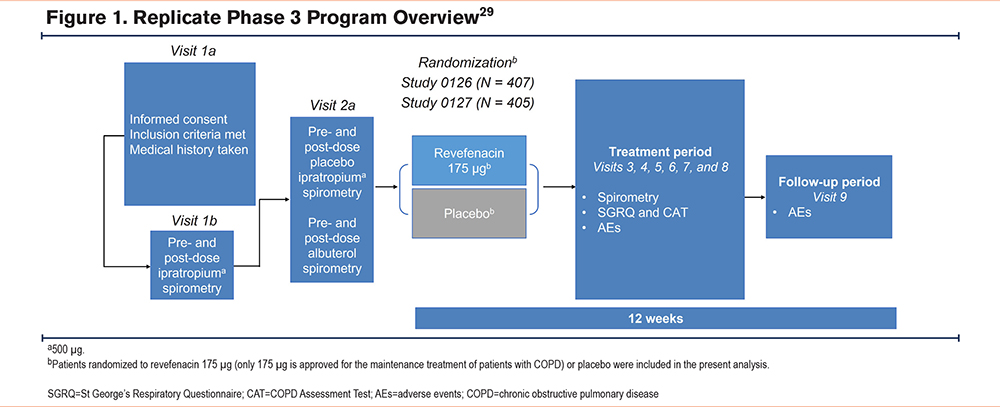
Patients self-identified psychiatric disorders during the data collection of their medical history. Medical history terms were mapped according to the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1. Psychiatric disorders were then categorized as anxiety and/or depression by expert (AMY, ASI, SPB) adjudication, and consensus was reached by discussion. Patients were grouped as having anxiety only (A), depression only (D), both anxiety and depression (+A/+D), or neither anxiety nor depression (−A/−D).
Assessments and Endpoints
Efficacy outcomes were evaluated in each of the 4 patient subgroups. The change from baseline in trough FEV1 (defined as the mean of the 23.25- and 23.75-hour spirometry assessments following the 84th dose) at Day 85 was evaluated. Following the final dose at Day 85, the percentage of patient responders, defined as the percentage of individuals having a ≥100-mL increase in postbronchodilator FEV1 within 2 hours of dose administration, was quantified.
This subanalysis assessed patient responses on the St George’s Respiratory Questionnaire (SGRQ)31 and the COPD Assessment Test (CAT).32 Endpoints were the change from baseline in SGRQ and CAT scores at Day 85. Responder analysis measured the percentage of patients with a minimum clinically important difference (MCID) in SGRQ (≥4-unit decrease from baseline) and/or CAT (≥2-unit decrease from baseline) scores at Day 85.
Safety assessments included treatment-emergent adverse events (TEAEs) and adverse events (AEs) commonly associated with antimuscarinic agents, including dry eye, dry mouth, dysuria, and constipation. The incidence of TEAEs and antimuscarinic AEs was compared across subgroups.
Statistical Analysis
Responses were assessed in the intention-to-treat population, defined as all patients who were randomized, received ≥1 dose of treatment, and had ≥1 postbaseline spirometry assessment. Least-squares (LS) mean changes from baseline in trough FEV1 at Day 85 and LS mean treatment differences between revefenacin 175 μg and placebo were estimated using a mixed model for repeated measures. LS proportions of responders at Day 85 were compared using a repeated-measures logistic regression model. The efficacy outcomes were adjusted for clinically significant variables that affect FEV1. LS mean changes from baseline in SGRQ and CAT scores at Day 85 and LS mean differences between revefenacin and placebo were estimated using a mixed model for repeated measures. The model included fixed effect class terms for treatment group, smoking status, reversibility status, concomitant LABA use at baseline, sex, and age at baseline. Odds ratio estimates and differences between revefenacin and placebo in the PRO responders were compared using a repeated-measures logistic regression model using similar covariates as above. Significance was set at P <0.05.
Results
Sociodemographic Characteristics
Across the two phase 3 trials, 1229 patients were randomized, of which a total of 266 discontinued the trial treatment period.29 For this exploratory post hoc analysis, of the 812 patients who were randomized to either revefenacin 175 μg or placebo, 90 (11%) had A, 110 (14%) had D, 141 (17%) had +A/+D, and 471 (58%) had −A/−D based on self-reporting (Table 1). The mean age was 59 to 66 years across subgroups. The mean baseline post-ipratropium percentage predicted FEV1 was 53% to 57%. A greater percentage of patients with A (68%), D (55%), or +A/+D (66%) were women compared with −A/−D (42%). A larger percentage of patients with +A/+D (64%) were smoking at baseline than those with A (53%), D (44%), or −A/−D (43%).
Lung Function
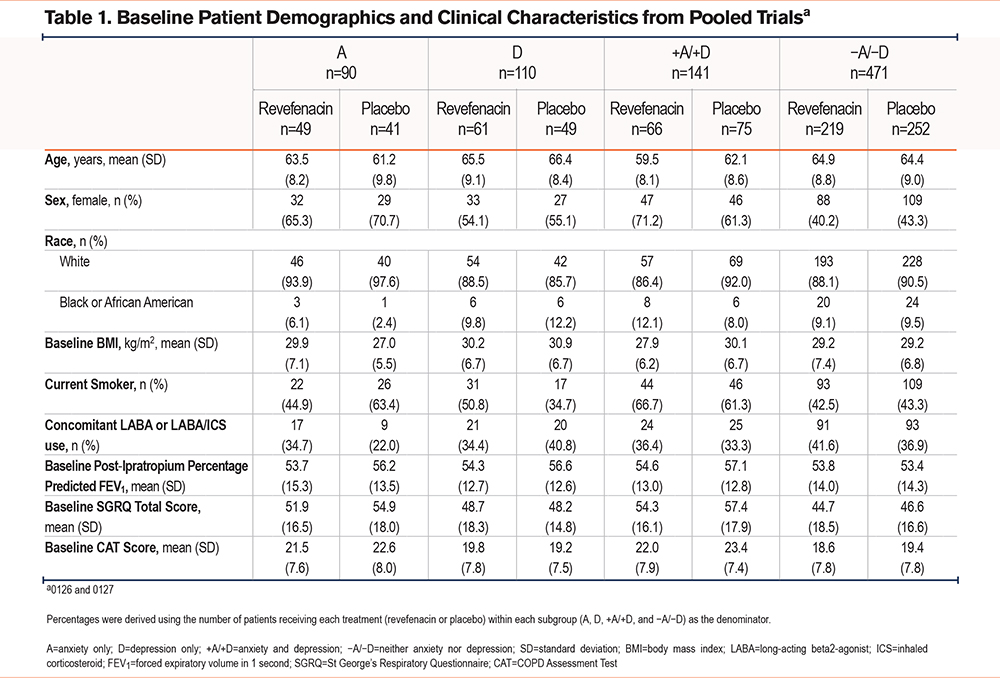
Revefenacin produced significant improvements from baseline versus placebo in trough FEV1 at Day 85 across all subgroups. The placebo-adjusted LS mean change (95% confidence interval [CI]; P-value) was 152 mL (55 to 250; <0.002) in patients with A, 111 mL (19 to 204; 0.02) with D, 150 mL (69 to 231; <0.001) with +A/+D, and 159 mL (116 to 202; <0.001) with −A/−D (Figure 2).
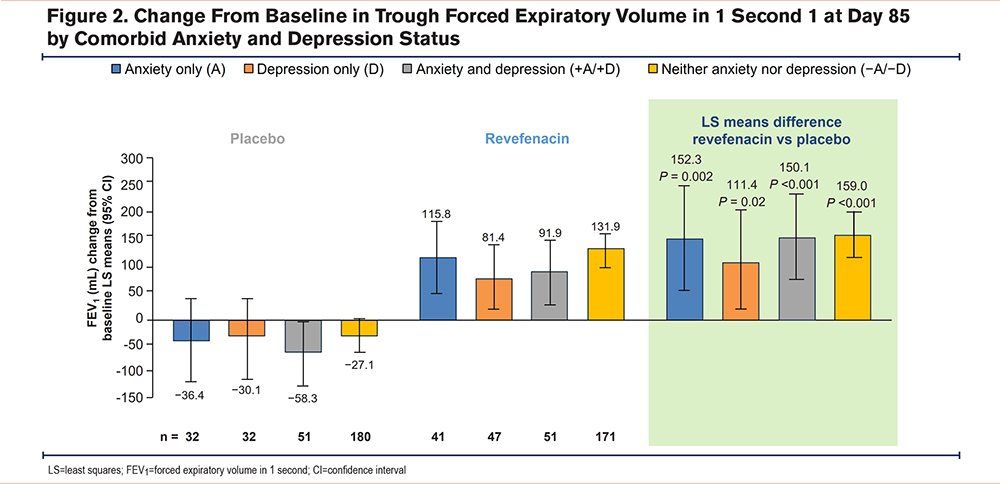
More patients who received revefenacin had a clinically relevant improvement of ≥100 mL from baseline in FEV1 versus placebo across all subgroups (Figure 3). The difference between revefenacin and placebo was not statistically significant in patients with D, potentially due to limited sample sizes.
St George’s Respiratory Questionnaire Score: Figure 4 shows that compared with placebo, revefenacin significantly improved total SGRQ scores (placebo-adjusted LS mean change [95% CI; P-value]) in patients with A (-8.2 [-13.2 to -3.1; 0.002]), +A/+D (-5.6 [-9.6 to -1.5; 0.007]), and −A/−D (-2.4 [-4.7 to -0.2; 0.03]). However, improvements in SGRQ scores were not significantly different for those with D (-0.9 [-5.5 to 3.7; 0.70]). SGRQ scores meeting the responder criterion for revefenacin and placebo were observed in 51% and 32% of patients with A, 36% and 33% with D, 60% and 40% with +A/+D, and 45% and 36% with −A/−D, respectively.
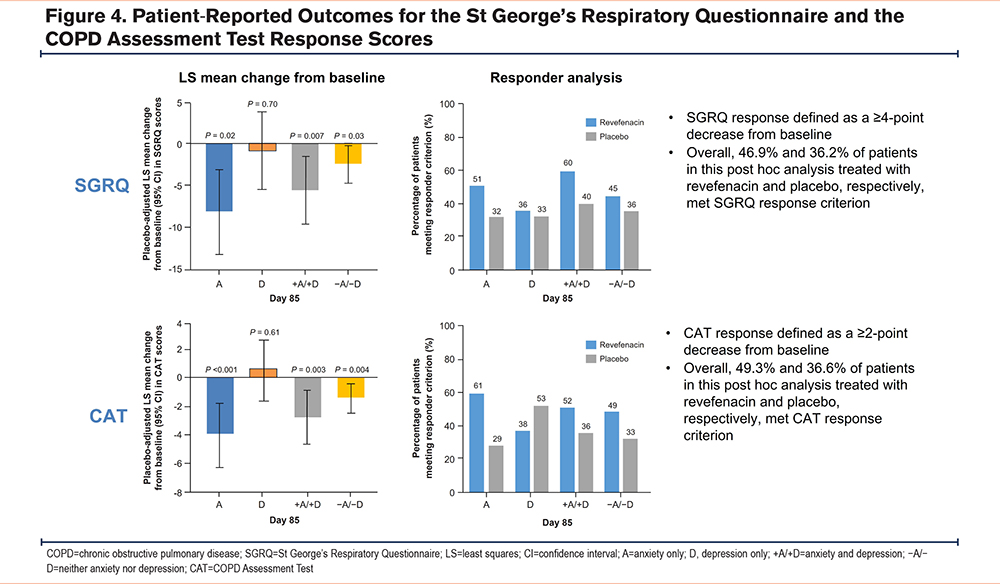
COPD Assessment Test Score: CAT scores (placebo-adjusted LS mean change [95% CI; P- value]) improved in patients with A (-4.1 [-6.5 to -1.8; <0.001]), +A/+D (-2.9 [-4.8 to -1.0; 0.003]), and −A/−D (-1.5 [-2.6 to -0.5; 0.004]). However, CAT scores were not significantly different for those with D (0.6 [-1.6 to 2.8; 0.61]). CAT scores meeting the responder criterion for revefenacin and placebo were observed in 61% and 29% of patients with A, 38% and 53% with D, 52% and 36% with +A/+D, and 49% and 33% with −A/−D, respectively, as shown in Figure 4.
Safety and Adverse Events
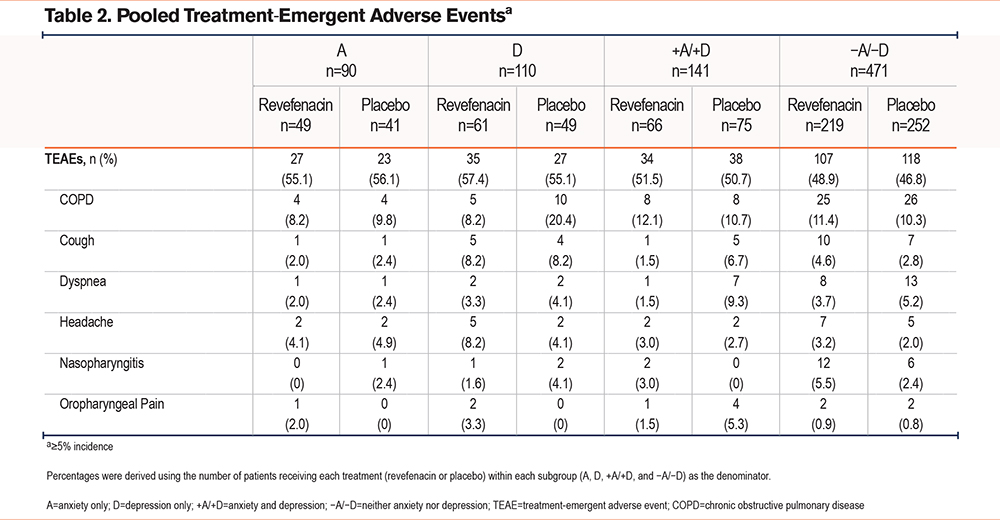
Across the 2 phase 3 trials, 180/812 (22.2%) patients from the randomized and treated analysis set discontinued the trial treatment period (72/395 [18.2%] revefenacin 175 µg and 108/417 [25.9%] placebo; discontinuation rates for the A/D subgroups were not recorded). In this exploratory post hoc analysis, AEs were reported in 56% of patients with A, 56% with D, 51% with +A/+D, and 48% with −A/−D. Revefenacin was generally well tolerated regardless of comorbid anxiety or depression status, with similar percentages of patients reporting TEAEs across subgroups. The incidence of treatment-emergent antimuscarinic AEs was minimal across all subgroups (Table 2).
Discussion
We examined the effects of revefenacin (a LAMA administered once daily for the maintenance treatment of patients with COPD) on lung function and QoL improvements in patients with COPD who also had self-reported anxiety and/or depression in an exploratory post hoc subgroup analysis from 2 randomized controlled trials. Compared with placebo, revefenacin achieved clinically significant improvements in lung function in the subgroups A, D, and +A/+D, with a treatment benefit similar to those without these comorbidities, −A/−D. Importantly, revefenacin showed clinically significant improvement in SGRQ and CAT scores in the A, +A/+D, and −A/−D subgroups when compared with placebo, but not in patients with D.
One in 6 patients self-reported comorbid +A/+D at baseline in these phase 3 clinical trials. These findings are similar to another exploratory analysis of subcategorized +A/+D patients with moderate-to-very severe COPD receiving nebulized glycopyrrolate 25µg administered twice daily, which resulted in numerical improvements in FEV1 and SGRQ total scores and responder rates irrespective of baseline A/D status.33 One difference between these treatments is that revefenacin administration is once daily, which reduces the treatment burden for patients with COPD versus twice-daily administration of glycopyrrolate. The prevalence of combined anxiety and depression in patients with COPD in these revefenacin phase 3 trials and the glycopyrrolate trials is approximately half the prevalence reported in an outpatient pulmonary rehabilitation program using clinically validated anxiety and depression outcome measures.8,9 This variation in anxiety and depression prevalence might be due to the self-reporting of +A/+D in the present revefenacin and prior glycopyrrolate studies, reflecting a patient’s reluctance or lack of comprehension regarding anxiety and depression unless specifically probed by health care professionals using validated anxiety and depression scales.34
Revefenacin showed significant improvement in trough FEV1 at Day 85 compared with placebo independent of anxiety and depression status (similar results were also demonstrated with glycopyrrolate administration).33 Although the clinical significance of lung function improvement observed (change from baseline in trough FEV1) in all subgroups of patients who received revefenacin versus placebo is unclear, these results contrast with the aforementioned glycopyrrolate study in which significant lung function improvements were only observed in the −A/−D group.33 The differences observed between the 2 studies may be related to trial design and the patient population.
The change in total SGRQ and CAT scores from baseline at Day 85 reached an MCID in only the A and +A/+D groups compared with placebo. Though statistically significant, improvements did not reach the MCID in the −A/−D group compared with placebo. This may be due to the differences in baseline SGRQ and CAT scores, which were higher in the A and +A/+D subgroups compared with the D and −A/−D groups. Caution is needed in the interpretation of SGRQ and CAT scores as studies generally require more than 12 weeks to show clinically meaningful differences with treatment, even when sufficiently powered. In addition, depression is linked to noncompliance with medical treatment.35 Although we did not evaluate adherence or compliance specifically in any of these subgroups due to the post hoc nature of this analysis, a combination of these factors could have contributed to the lack of improvement in SGRQ and CAT scores. Of note, in the 2 phase 3 trials of revefenacin, medication adherence rates were high (more than 90% of patients had adherence rates of 80% or more).29 These high adherence rates to revefenacin are notable given the high medication burden, regimen complexity for patients with COPD, and managing symptom load of comorbidities.36
The safety profile of revefenacin was similar to placebo in patients with COPD with and without comorbid anxiety and/or depression. This is consistent with previously reported data from the 2 phase 3 trials of revefenacin.29,30
Revefenacin is a tertiary amine distinct from other LAMAs, such as glycopyrrolate and tiotropium, which are quaternary ammoniums.30 The unique structure and the long dissociation half-life from the M3 receptor allow revefenacin to produce sustained (long duration of action) bronchodilation, with fewer antimuscarinic side effects.30,37-39 The results of our exploratory post hoc analysis describe the efficacy and safety of revefenacin in a specific population of patients with COPD (those with comorbid anxiety and/or depression). A previous post hoc subgroup analysis of these same phase 3 trials investigated the efficacy of revefenacin in patients with markers of more severe COPD and comorbidity risk factors (such as a history of cardiovascular disease, diabetes, and cognitive/mental impairments).40 In both post hoc analyses, LS mean differences in Day 85 trough FEV1 favored patients who received revefenacin versus placebo across subgroups. This benefit of revefenacin was also observed when examining SGRQ in all subgroups, except for patients with depression alone and patients over the age of 75. Overall, the significant improvement in lung function combined with the tolerability afforded by revefenacin in this exploratory post hoc analysis examining comorbid anxiety and/or depression subgroups provides useful information to health care professionals when assessing and treating these specific subpopulations of patients with COPD.
Strengths of this post hoc analysis include the large sample size drawn from 2 pivotal phase 3 trials of revefenacin in comparison with placebo on a background of standard of care, including 37% of patients using LABA (with or without inhaled corticosteroids) therapy. In addition, and in contrast to the previously mentioned glycopyrrolate study,33 an expert, independent blinded panel was utilized for confirmation of self-reported anxiety and depression symptoms from medical notes. This exploratory post hoc analysis has several limitations. First, this was not a prespecified subgroup analysis, and information on patient use of antidepressants or antianxiety medications was not gathered in a systematic way. Therefore, these results should be considered hypothesis-generating. Although an independent expert-blinded panel confirmed patient anxiety and/or depression status (comorbidities were first identified by patient self-reports and were matched with the MedDRA preferred terms), the use of validated anxiety and depression scales may have resulted in the detection of more individuals with anxiety and/or depression in this post-COVID-19 era given the complex mental health needs of patients with COPD, as well as other chronic diseases.41 Additionally, findings may not be generalizable to patients with COPD with mild respiratory impairments given the moderate-to-severe COPD population recruited to these trials. Finally, caution is needed regarding the interpretation of these findings as there was no examination of treatment adherence or discontinuation rates in this post hoc analysis.
Conclusions
In patients with moderate-to-very severe COPD, revefenacin produced significant improvements in trough FEV1 at Day 85 compared with placebo, independent of anxiety and/or depression status. In addition, revefenacin improved SGRQ and CAT scores in patients with COPD who also reported comorbid A, +A/+D, and –A/–D in this exploratory post hoc analysis. QoL improvements in the D subgroup were not statistically significant. Along with the high levels of adherence demonstrated in the parent phase 3 trials, combined with the simplicity of administration, the findings of this subanalysis support the use of revefenacin in patients with COPD who have comorbid conditions such as anxiety and/or depression.
Acknowledgements
Author contributions: All authors conform to the International Committee of Medical Journal Editors guidelines for authorship, substantially participated in the creation of the submitted work, significantly contributed to and approved the final version of the manuscript and take responsibility for the integrity of the work. AMY contributed to methodology, data analysis, writing the original draft, and writing and reviewing/editing the manuscript. ASI contributed to the conceptualization and writing and reviewing/editing of the manuscript. CCcontributed to conceptualization, data curation, formal analysis, writing the original draft, and writing and reviewing/editing the manuscript. LC contributed to conceptualization, data curation, formal analysis, project administration, and writing and reviewing/editing the manuscript. XC contributed to validation, visualization, and writing – reviewing/editing the manuscript. DAL contributed to conceptualization, formal analysis, and writing and reviewing/editing the manuscript. SPB contributed to the investigation and writing and reviewing/editing of the manuscript.
Editorial support was provided by Gregory Suess, PhD, CMPP, of AlphaBioCom, a Red Nucleus company, and was funded by Theravance Biopharma U.S., Inc., San Francisco, California.
Data sharing statement: Theravance Biopharma (and its affiliates) will not be sharing individual de-identified patient data or other relevant study data documents at this time.
Declaration of Interests
Abebaw M. Yohannesreports receiving support for attending meetings and/or travel from Theravance Biopharma. Anand S. Iyerreports receiving grants from the National Institutes of Health’s (NIH) National Institute on Aging, consulting fees from AstraZeneca, and speaking fees from Ascension. Candice Clayreports being an employee of Verona Pharma and a former employee and stock owner of Theravance Biopharma at the time of the study. Lauren Cochranreports being an employee and stock owner of Theravance Biopharma. Xianyi Chenreports being an employee of Theravance Biopharma. David A. Lombardireports being a contract employee of Theravance Biopharma at the time of his contribution as the project statistician. Surya P. Bhattreports receiving grants from the NIH, consulting fees from Sanofi, Regeneron, and Boehringer Ingelheim, and participation in a data safety monitoring board for the NIH.