Running Head: Physical Position Effect on Peak Inspiratory Flow
Funding Support: This research was funded by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy, as it relates to BIPI substances as well as intellectual property considerations.
Date of Acceptance: December 12, 2023 | Publication Online Date: January 17, 2024
Abbreviations: %pred=percentage predicted; BD=bronchodilator; BMI=body mass index; CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; DPI=dry-powder inhaler; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; IQR=interquartile range; mMRC=modified Medical Research Council; PIF=peak inspiratory flow; pMDI=pressurized metered‑dose inhaler; R2=low-medium resistance; R5=high resistance; SD=standard deviation; UNC-CH=University of North Carolina at Chapel Hill
Citation: Pleasants RA, Henderson AG, Bayer V, Shaikh A, Drummond MD. Effect of physical position on peak inspiratory flow in stable COPD: an observational study. Chronic Obstr Pulm Dis. 2024; 11(2): 174-186. doi: http://doi.org/10.15326/jcopdf.2023.0460
Online Supplemental Material: Read Online Supplemental Material (109KB)
Introduction
Chronic obstructive pulmonary disease (COPD) represents one of the top 3 causes of death globally,1 affecting approximately 380 million people.2 It is a major public health challenge1 with a significant economic burden2 that is projected to increase primarily because of continued exposure to COPD risk factors and population aging.3 Improved long-term management of COPD is key to reducing mortality and morbidity, as well as lowering the economic impact, which is primarily related to hospital admissions for exacerbations.4
The primary treatment modalities for COPD after smoking cessation and environmental/occupational exposure avoidance include the administration of inhaled medications. These medications can be delivered through multiple mechanisms, including pressurized metered-dose inhalers (pMDIs), dry-powder inhalers (DPIs), soft mist inhalers, or nebulizers.3 DPIs, which account for more than 35% of the global inhaler market,5 are breath-actuated devices with varying levels of internal resistance.6 While many factors may impact the effective delivery of medication to the lungs with a DPI, users must overcome the internal resistance of the DPI by achieving peak inspiratory flow (PIF), defined as the maximal flow rate, typically expressed in L/min, obtained during an inspiratory maneuver.6 Low PIF may negatively impact drug delivery to the lungs via DPIs7 and has been associated with poor patient outcomes8 and higher COPD-related health care utilization and costs.9
Practical and standardized recommendations for measuring PIF in patients with COPD using DPIs are lacking,6 including whether the patient’s physical position affects the maximal inspiratory effort achieved. Previous studies have shown the association of modifiable factors with improved pulmonary function,6,8,10 including PIF assessed by spirometry or portable inspiratory devices; however, there remains a paucity of information about the modifiable factors affecting PIF in patients with COPD. Moreover, despite the evidence that physical position may influence pulmonary function,11 the association of PIF with physical position during DPI use in patients with COPD remains unknown. The effect of physical position on maximum inspiratory effort may be most significant during severe exacerbations when patients’ lung function is severely impaired, and they often receive inhaled medications in the semirecumbent position.
In a group of stable outpatients with COPD, we investigated whether different physical positions, specifically, the semi-upright position commonly used in acute care settings and the sitting and standing positions typically used in the ambulatory setting, affected PIF (In-CheckTM DIAL, Clement Clarke International, United Kingdom). To the best of our knowledge, this has not been reported previously.
Methods
Study Design
Data for this exploratory analysis were extracted from a single visit occurring as part of a larger prospective, observational, 24-week study conducted in stable, ambulatory adults with COPD using a DPI (NCT04168775). The University of North Carolina at Chapel Hill (UNC-CH) Institutional Review Board reviewed and approved the protocol, informed consent forms, and other relevant documents (IRB# 19-0450). All participants provided written informed consent.
Participants
Adults aged >50 years with COPD seen at the UNC-CH outpatient pulmonary clinics who were either current or former smokers (>10 pack-years of smoking) were eligible to participate. Other eligibility criteria were as follows:
- spirometry-confirmed diagnosis of COPD (postbronchodilator forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC] <0.70)
- COPD at Global initiative for chronic Obstructive Lung Disease (GOLD)1 stages 2–4 based on existing spirometry results
- COPD Assessment Test (CAT) score >10
- ≥ 1 COPD exacerbation requiring systemic corticosteroids within the last 3 years
- stable COPD (no recent exacerbation in the past 30 days)
- PIF of 60–90 L/min against low-medium resistance (R2) DPIs (e.g., Diskus®, Ellipta®) or 30–90 L/min against high resistance (R5) DPIs (e.g., Handihaler®), depending upon the prescribed maintenance inhaler device using the In-CheckTM DIAL to assess PIF.12
The key exclusion criteria were inability to demonstrate proper technique for the In-CheckTM DIAL device, inability to achieve minimum PIF rate for the prescribed DPI(s) at the screening/enrollment visit (e.g., <30 L/min, for Handihaler® [high-resistance DPI], <60 L/min for Ellipta® [medium-resistance DPI]), and neuromuscular disease associated with weakness.
Peak Inspiratory Flow Measurement
PIF was measured for each participant in 3 positions: standing, sitting, and semi-upright (supine with the head of the bed at 45° and their neck flexed forward), and all measurements were taken at the same visit. Measurements were taken in a random order with regard to the physical position. For each physical position, PIF was measured in triplicate with the highest of the 3 measurements used for analysis.6 The In-CheckTM DIAL is a handheld respiratory flow–measurement device designed to simulate the resistance of inhaler devices from the DPI and metered-dose inhaler categories, which enables clinicians to coach patients to use more or less inspiratory flow to achieve optimal flow rates.13 The In-CheckTM DIAL was set according to the manufacturer’s recommendations using the resistance of the inhaler(s) that the patients were using at home (R2, for patients who were using R2 resistance; R5, for patients who were using R5; or both, for patients who were using both R2 and R5 inhalers). The participants’ bronchodilators were withheld prior to the visit and positional PIF measurements were performed before bronchodilator (BD) reversibility testing with spirometry. Pre and postspirometry were performed concurrent to pre- and post-PIF measurements, with each test done in at least triplicate to meet the American Thoracic Society/European Respiratory Society spirometry guidelines.14 In contrast to R2 and R5 resistance settings on the In-CheckTM DIAL device, the resistance to inhalation is essentially zero with a spirometer. Participants were directed with each PIF measurement to follow the manufacturer’s instructions for the In-CheckTM DIAL by exhaling slowly and completely, followed immediately by a rapid (“as fast as possible”) and maximal inhalation (“until you cannot inhale anymore”).6 If a patient was using both R2 and R5 DPIs, both R2 and R5 positional measurements contributed to the data. Positional PIF measurements were taken at a single time point during the study, either at week 1 (enrollment) or week 24. These time points were selected due to the impact of the SARS-CoV-2 virus on the study design.
Participants were asked at the outset of the study about how they most commonly administered their DPIs with respect to physical position when at home; the screening of PIF for study eligibility was performed based on each participant’s reported physical positions at home. We also measured the waist-to-hip ratio15 to determine whether central adiposity might influence PIF among the different positions. For post-BD spirometry measurements, the BD was administered as 4 puffs of albuterol with a spacer, and lung function tests were repeated 15–20 minutes later.
Statistical Analysis
For this analysis, it was hypothesized that PIF would be reduced in patients in the semi-upright position versus the sitting and standing positions. This was an exploratory analysis, and consequently, no formal power calculations were conducted. Descriptive statistics were used to summarize the clinical and pulmonary baseline function characteristics. Data are presented as mean (standard deviation [SD]), median (interquartile range [IQR)]), or n (%). Baseline demographic, clinical, and pulmonary characteristics were stratified by inhaler device type at enrollment; however, no statistical analyses were performed as the groups were not assigned a priori. Differences in PIF between positions were assessed using a paired t‑test (p-values were not corrected for multiple comparisons). The normality of distributions was assessed using quantile-quantile plots and the Shapiro-Wilk test. The percentage decline in the positions for each DPI type was calculated as follows:
- Standing to sitting = [(PIFstanding − PIFsitting) / PIFstanding] × 100;
- Standing to semi-upright = [(PIFstanding − PIFsemi-upright) / PIFstanding] × 100;
- Sitting to semi-upright = [(PIFsitting − PIFsemi-upright) / PIFsitting] × 100.
The demographic and clinical characteristics of participants with a >10% and ≤10% PIF decline from standing to semi-upright positions were compared and are presented as mean (SD) or n (%) and associated standardized difference, which is a qualitative description of differences. There is no known minimum clinically important difference for PIF. The 10% threshold was selected because it was outside the reported variation of the In-CheckTM DIAL and aligned with the magnitude of change observed in bronchodilator testing.6,13 An absolute standardized difference of ≥0.10 or ≤−0.10 indicates a potential imbalance in covariates between groups.16
The distribution of PIF data is graphically presented using box and whiskers and scatter plots. The Spearman correlation coefficients of PIF for pairs of physical positions were calculated. All analyses were independently completed by one of the authors (MBD) using Stata version 16.1 (StataCorp LLC, College Station, Texas).
Results
Participants
Between July 31, 2019, and November 9, 2021, 96 participants were screened for the study and 80 were subsequently enrolled, with 76 participants contributing positional PIF data. Of the excluded patients, 10 did not achieve PIF in the target range, while the remainder did not use the prescribed DPI or had a CAT score <10. The baseline demographic, clinical, and pulmonary characteristics of participants with positional data are shown in Table 1. The mean (SD) age was 65.2 (8.77 years) years. A total of 42 (55%) participants were female, most were White (n=66; 87%), and 27 (36%) and 49 (64%) were current and former smokers, respectively. The mean (SD) body mass index (BMI) was 28.8 (7.26) kg/m2; 28 (36.84%) patients were obese (BMI ≥30 kg/m2) and 4 (5%) were severely obese (BMI ≥40 kg/m2). When using a DPI at home, 45 (59%) participants reported normally using a sitting position and 31 (41%) a standing position. The mean predicted pre- and post-BD FEV1 was 45% and 48.7%, respectively. The mean (SD) pre-BD PIF was 182 (67.2) L/min (by spirometry) and 69 (15.4) L/min (by In-CheckTM DIAL), and 179 (72.8) L/min (by spirometry) and 75.3 (16.6) L/min (by In-CheckTM DIAL) post-BD. A total of 57 participants (75%) were using R2 DPIs only at home, 13 (17%) were using R5 DPIs only, and 6 (8%) were using both. Overall, patients using R5 DPIs were younger and had a lower BMI, higher CAT score, and lower lung function compared with those using R2 DPIs alone or R2 and R5 DPIs (Supplementary Table 1 in the online supplement).
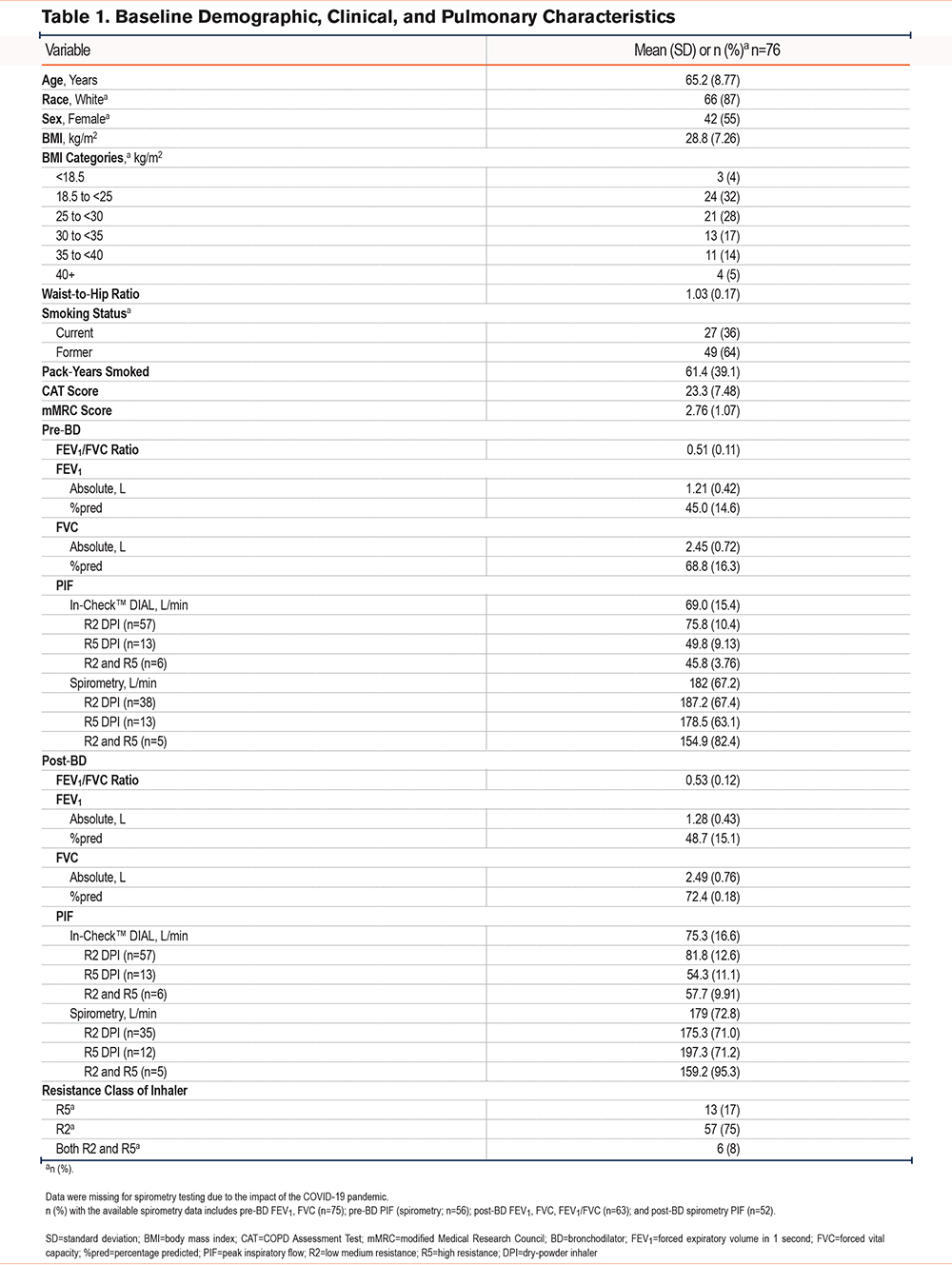
Correlation of Peak Inspiratory Flow for Physical Positions
The median (IQR) PIF for participants in each physical position using the R2 and R5 DPIs is shown in Figure 1. For participants using R2 DPIs, the median (IQR) PIF in the standing, sitting, and semi-upright positions was 80 (70–88), 78 (66–85), and 75 (63–80) L/min, respectively, and the mean (SD) PIF was 80.7 (13.4), 77.8 (14.3), and 74.0 (14.5), respectively. For participants using R5 DPIs, the median (IQR) PIF in the standing, sitting, and semi-upright positions was 50 (47–59), 50 (43–56), and 46 (39–52) L/min, respectively, and the mean (SD) PIF was 51.1 (9.52), 48.6 (9.84), and 45.8 (7.69) L/min, respectively. In all 3 physical positions, the mean and median PIF for participants using an R2 DPI was higher than that for participants using an R5 DPI. For participants using R2 DPIs, the PIF in the semi-upright position was significantly lower than that in the sitting and standing positions ( P< 0.0001). For participants using R5 DPIs, the PIF in the semi-upright position was significantly lower than that in the standing position (P=0.002). All positional measurements for both the R2 and R5 DPIs were normally distributed (data not shown).
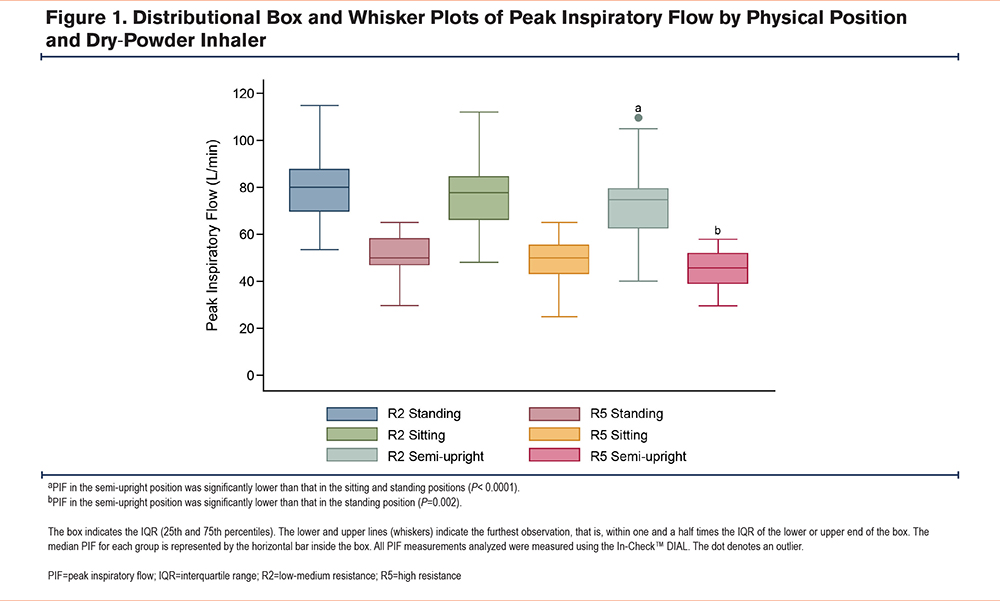
The correlation of PIF for pairs of physical positions (standing versus sitting, standing versus semi-upright, and sitting versus semi-upright) for participants using R2 and R5 DPIs is shown in Figure 2. In all physical positions, the PIF for participants using an R2 DPI was higher than that for participants using an R5 DPI. The PIF correlations for participants using R2 DPIs were as follows: standing versus sitting, Spearman’s rho 0.875 (P < 0.0001); standing versus semi-upright, Spearman’s rho 0.887 (P < 0.0001); and sitting versus semi-upright, Spearman’s rho 0.888 (P < 0.0001). Furthermore, the PIF correlations for participants using R5 DPIs were as follows: standing versus sitting, Spearman’s rho 0.7132 (P=0.0006); standing versus semi-upright, Spearman’s rho 0.717 (P=0.0005); and sitting versus semi-upright, Spearman’s rho 0.682 (P=0.0013).
Owing to changes in visit structure as a result of the COVID-19 pandemic, a total of 29 participants (38% of the analytical cohort) had positional data measured at the enrollment visit, while 47 participants (62% of the analytical cohort) had positional data measured at study completion (week 24). On reviewing the PIF values stratified by timing of positional data collection, no consistent and meaningfully higher PIF values among those completing measurements at the final study visit were observed, suggesting no impact of training effect on the study findings.
Peak Inspiratory Flow Change With Physical Position
The number (%) of participants with a >10%, >15%, and >20% decline in PIF between pairs of physical positions was calculated for R2 and R5 DPIs, and the percentage decline in PIF is shown in Table 2. Approximately half of the participants had a >10% decrease in PIF from the standing to semi-upright positions for R2 (n=31; 50%) and R5 (n=10; 53%) DPIs, and 11 (18%; R2) and 6 (32%; R5) participants had a >10% decrease in PIF from the sitting to semi-upright positions. A small percentage of participants had a decline of >15% or >20% for both R2 and R5, with the largest proportion in the standing to semi-upright positional change. Two participants using R5 DPIs and 1 patient using an R2 DPI reported a >50% difference between standing and semi-upright positions.
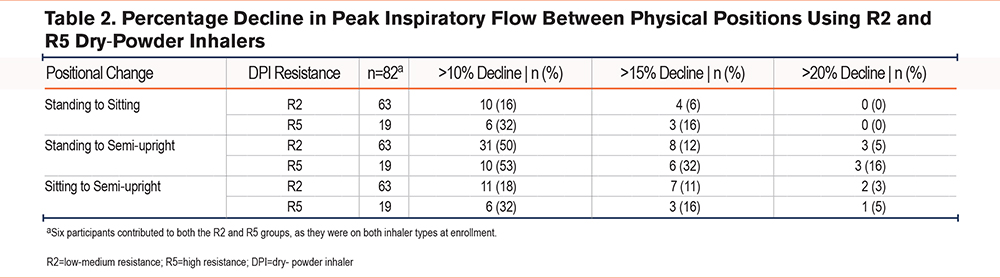
Of note, the proportion of patients using R2 DPIs who did not reach optimal PIF (<60 L/min) for R2 were 1.3% (n=1 of 76), 6.6% (n=5 of 76), and 10.5% (n=8 of 76) for the standing, sitting, and semi-upright positions, respectively. All patients using R5 DPIs reached optimal PIF (>30 L/min).
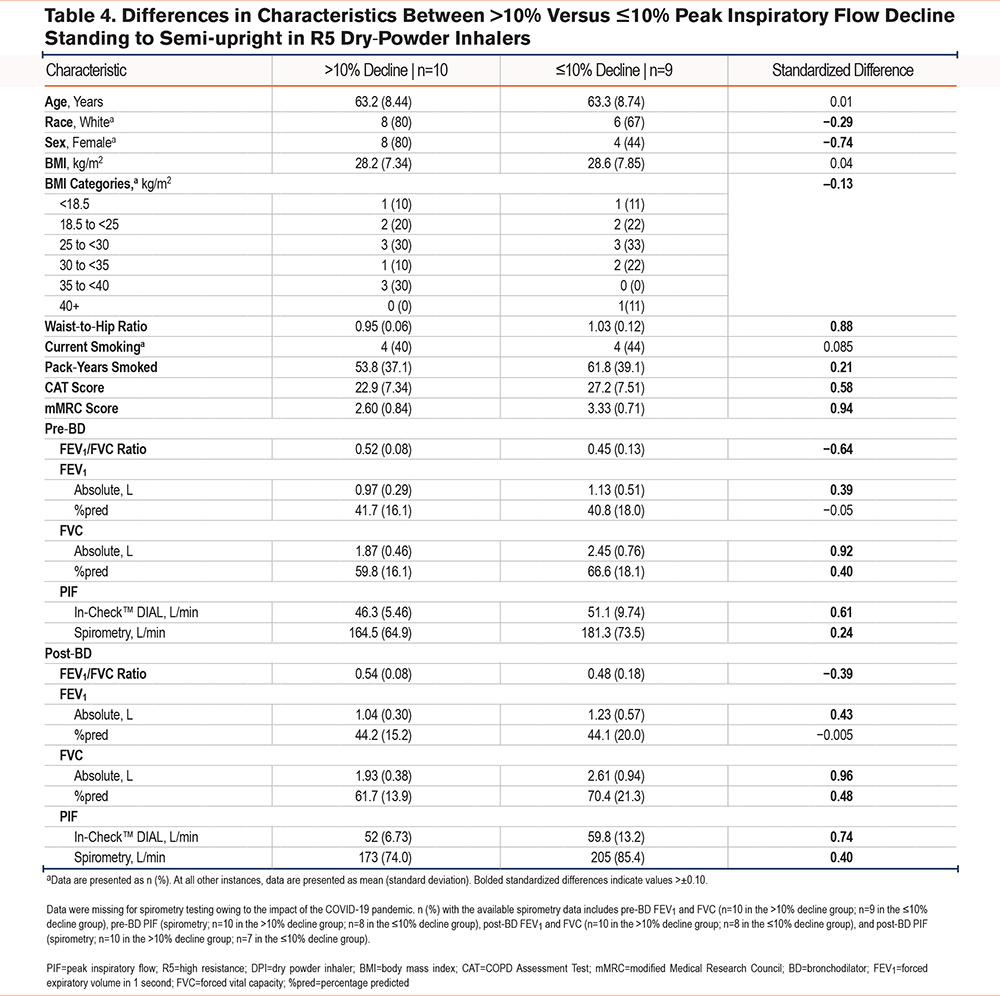
Differences in the Demographic and Clinical Characteristics in Participants With Peak Inspiratory Flow Decline >10% versus ≤10% from the Standing to Semi-upright Positions
We examined the differences in demographic and clinical characteristics between participants with a decline in PIF from the standing to semi-upright positions (>10% versus ≤10%) as seen in Table 3 and Table 4. This threshold was also chosen because it was outside the reported variation of the In-CheckTM DIAL and aligned with the magnitude of change observed in bronchodilator testing. Approximately half of the participants using R2 or R5 DPIs exhibited a >10% decline in PIF from the standing to semi-upright positions (50% and 53%, respectively). The mean BMI did not differ between patients with a >10% decline versus those with a ≤10% decline when standing to semi-upright using an R2 or R5 device, although there was a greater proportion of obese patients (BMI: ≥30 kg/m2) in the >10% versus ≤10% group among those using R5 DPIs (40% versus 33%). A >10% decline in patients using R2 or R5 DPIs was associated with a lower waist-to-hip ratio compared with those who had a ≤10% decline.
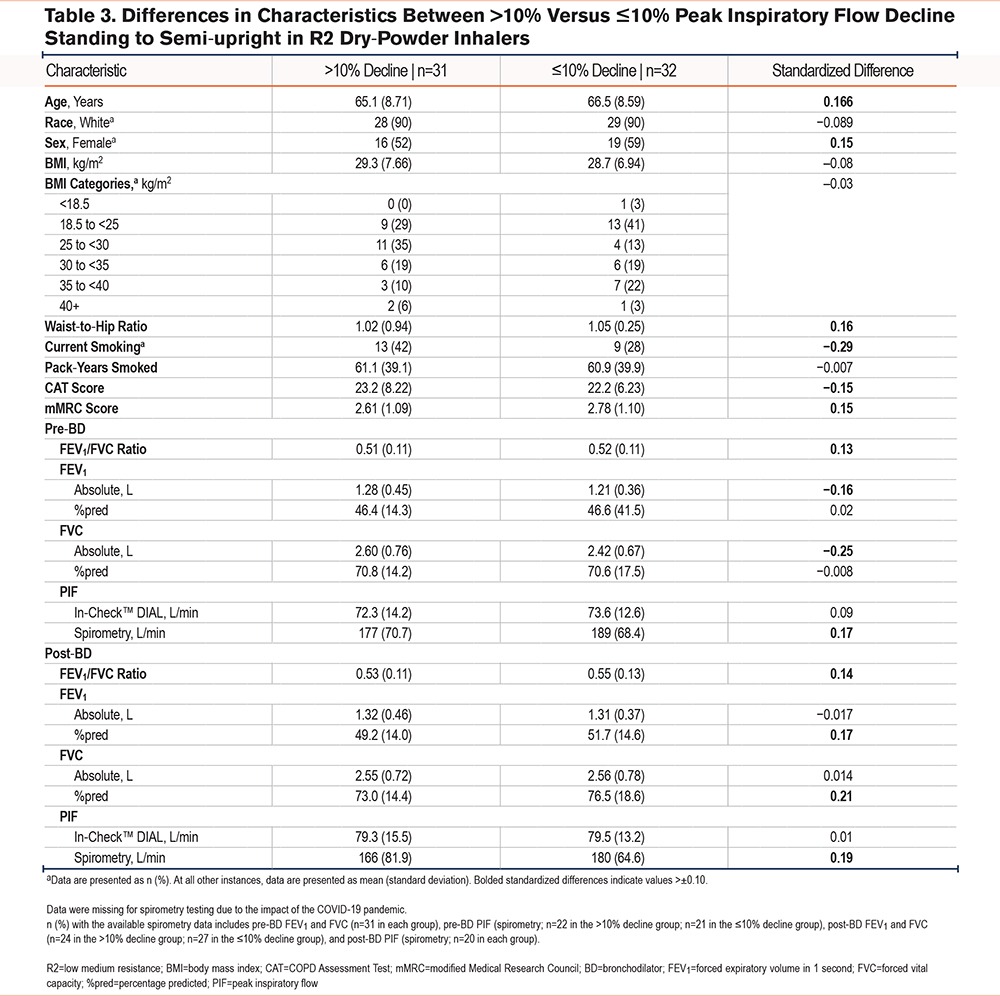
Several clinical characteristics exceeded the 0.10 absolute standardized difference threshold with the decline in PIF using R2 DPIs, including age, sex (female), waist-to-hip ratio, modified Medical Research Council (mMRC) score, pre-BD FEV1/FVC ratio and PIF by spirometry, post-BD FEV1/FVC ratio and PIF by spirometry, percentage predicted (%pred) FEV1, and %pred FVC. The characteristics that had their absolute standardized threshold for R2 DPIs below the −0.10 threshold were current smokers, CAT score, pre-BD absolute (L) FEV1, and absolute (L) FVC (Table 3).
Participant characteristics that exceeded the 0.10 standardized difference threshold with R5 DPIs included waist-to-hip ratio, pack-years smoked, CAT score, mMRC score, pre- and post-BD absolute (L) FEV1, absolute (L) FVC, %pred FVC, and PIF by spirometry and In-CheckTM DIAL. Characteristics that had their absolute standardized threshold for R5 DPIs below the −0.10 threshold were race (White), sex (female), higher BMI obesity category, and pre- and post-BD FEV1/FVC ratios (Table 4).
Discussion
This study showed for the first time in a stable COPD population that PIF measurement using an inspiratory flow meter is affected by the patient’s physical position. Mean PIF was the highest in the standing position and lowest in the semi-upright position. Approximately half of the participants had a >10% decline in PIF from the standing to semi-upright position, and nearly one-third exhibited a ≥15% decline in PIF with the higher resistance setting. Several demographic and clinical characteristics were imbalanced among participants with a PIF decline >10% versus ≤10% from standing to semi-upright, including sex (female), FVC, and smoking status. These findings have important implications for guiding patients on optimal techniques to maximize PIF when using DPIs by reinforcing the importance of standing or sitting (where possible), rather than adopting a semi-upright position. This is especially true during exacerbations, as PIF and other inspiratory lung function measures are known to be substantially reduced.17 We also report, for the first time, the typical physical positions adopted by individual patients with COPD when self-administering their DPIs at home.
In the current study, the PIF for participants using the R2 DPI was higher than that for participants using the R5 DPI in all physical positions. There was a strong correlation between standing, sitting, and semi-upright measurements of PIF for participants using R2 and R5 DPIs. Dal Negro examined whether DPIs at different intrinsic resistance regimens (low, medium, and high) were affected by changes in lung function and/or could be predicted by specific lung function parameters in patients with asthma, COPD, or restrictive lung disease.18 The study showed that patients with asthma or COPD using medium-resistance DPI regimens were more likely to achieve the expected PIF level than those using low- or high-resistance DPI regimens. Thus, DPI resistance can significantly influence PIF.19 Our study also suggests that DPI resistance, in combination with the physical position adopted when using a DPI, may impact PIF.
When using a DPI at home, 45 (59%) participants reported using a sitting position and 31 (41%) reported using a standing position. We assume that this preference for sitting or standing may apply to other inhalers as well. Our findings can inform instructions given to patients using an R2 or R5 DPI, recommending that they stand when possible. Given that this approach may not be possible in hospitals or nursing homes due to safety and convenience concerns, or at home due to safety and physical impairment concerns, the recommended position should be sitting rather than lying down (semi-upright) if possible. Furthermore, in cases where the PIF is significantly impaired, the use of a wet aerosol inhaler or nebulizer may be preferable. While decreased efficacy due to suboptimal PIF with DPIs has been reported,20 the impact of low inhalational flow rates and subsequently increased oral deposition on oropharyngeal side effects of inhaled corticosteroids remains unclear. A real-world study reported that patients using fluticasone propionate/salmeterol DPIs were more likely to report oral candidiasis than those using the pMDI formulation.19
Prior studies employing spirometry have examined the effects of physical position on expiratory flow measurements.17,21,22 The position of the head and its relationship with airflow in respiration was described23 as early as 1959. Harris found a statistically significant difference in the forced inspiratory volume achieved within the first 0.5 seconds when the head was flexed compared with when it was fully extended.23 Numerous recent studies have shown that gross postural changes affect pulmonary function, with standing and sitting leading to the highest lung volumes.11 Physical position changes have been shown24 to affect individuals with normal lung function, with a mean decrease in FVC from sitting to supine of around 7%. Decreased maximum inspiratory pressure, which correlates well with PIF in COPD,25 observed in the semi-upright position could be related to diaphragm overload by abdominal content displacement during maximal inspiratory effort.11 This effect may offset the improved diaphragm function associated with the position, allowing for an optimal length-tension relationship in the diaphragmatic muscle fibers. Furthermore, the lengthening of other inspiratory muscles may become restricted in the semi-upright position.11 Indeed, the “tripod position,” a seated position where the trunk is leaning forward with the arms braced on the knees, is adopted by individuals to relieve dyspnea in severe COPD.10,26 Studies have shown that this position enables accessory muscles, such as the pectoralis major and minor, to significantly contribute to rib cage elevation.27 Additionally, adopting a seated, forward‑leaning posture is associated with an improved ability to generate maximal inspiratory pressure, improved length-tension relationships and neuromechanical efficiency of the diaphragm, and reduced neuromechanical dissociation of the respiratory system.28 Lifting the chin while using DPIs is often recommended for more efficient passage of the drug into the lungs; this may not be possible in the semi-upright position. In the current study, participants who were in the semi-upright position at 45° with their head held forward to where the neck was near vertical (more flexed than extended) had lower PIF than those in the standing or sitting position. Moreover, we found that approximately half of the participants had a >10% drop from the standing to semi‑upright position. A small number of participants showed large differences between these 2 positions. No clear, minimally important clinical difference has been established for PIF. While this study was not designed to correlate the impact of positional changes in PIF to clinical outcomes or drug delivery, these findings suggest that positional modifications (i.e., standing rather than being semi-upright or sitting) may be an approach to improve PIF.
Body habitus (obesity and waist-to-hip ratio) could have a differential impact on PIF based on participant position. In this study, we did not observe a difference in the mean BMI between those with a >10% decline and those with a ≤10% decline from the standing to semi-upright positions. Therefore, it does not appear that obesity or central adiposity, when combining males and females, were substantial drivers in the observed positional changes in PIF.
Achieving an adequate PIF with DPIs is considered vital for optimizing aerosol drug delivery in the COPD population. While a maximal inhalation is desirable to disaggregate the dry powder in the inhaler to achieve optimal particle size, it is possible that excessively rapid inhalation with a DPI might increase oropharyngeal deposition. Studies comparing aerosol delivery with the same DPI at rapid versus slow, forceful inhalation and resultant oropharyngeal deposition have not been reported to our knowledge. One study in children found that more rapid (higher) inhalation rates resulted in a greater improvement in FEV1.29 Additionally, differences in instructions on proper inhalation technique for DPIs with the same degree of internal resistance do exist (e.g., Diskus® inhaler30 instructions recommend deep, rapid inhalation while instructions for the Ellipta® inhaler31 recommend full, complete inhalation). However, an expert group recommends a deep, forceful inhalation for DPIs.6
This is the first study to measure PIF in different physical positions in a standardized manner, and to the best of our knowledge, there is little data published on this topic. The recommendations from our study are straightforward and practical to implement. Ideally, patients should have their PIF measured, but it is often not performed in routine clinical practice. Consideration of physical position is an easily modifiable factor that can be realistically performed by patients in a clinical or home setting with proper guidance. Furthermore, our approach of randomizing the sequence of positions of PIF measurements decreased the chances of individual training effects during repeated measures.
The current study has some limitations. The sample size and single-center setting of the study may limit the generalizability of the results; moreover, being an observational study, it may be prone to bias. The timing of PIF measurements and spirometry, which was impacted by the COVID-19 pandemic, occurred at 2 different time points in the study (some at the start of the study and some at the end), and, therefore, may have introduced variability into the data; however, our findings suggest no consistent or meaningful impact of the timing of positional data collection on study results. It is also possible that patients with prior experience with the In-CheckTM DIAL inspiratory flow meter use may have performed differently than those naïve to the device. Additionally, the PIF measured within the study setting may be higher than that recorded in real-life settings, as participants were performing inhalation maneuvers under supervision. Follow-up studies could include measurements of pulmonary function, including lung volume, which would further inform the findings of the current study. Additional clinical trials examining PIF measurements in hospitalized patients who are positioned semi-upright and receive DPIs versus no DPIs may be warranted.
Conclusions
In a stable COPD population, PIF was the lowest in the semi-upright position, regardless of the resistance and type of DPI used. This study highlights the importance of the physical position adopted during the DPI inhalation maneuver and in measuring PIF in patients with COPD. Our findings provide further evidence in favor of standardizing the physical positioning during DPI use to ensure adequate drug delivery to the lungs and superior patient outcomes. The findings of the current study may inform instructions given to patients with COPD when using DPIs, especially in a hospital, nursing home, or an outpatient setting. Previously unreported, almost 60% of participants indicated that they normally used their DPIs while sitting. It is recommended that patients stand while using an R2 or R5 DPI when possible. When this approach is not possible, the recommended position should be sitting rather than semi-upright.
Acknowledgements
Author contributions: RAP and AGH, substantially contributed to the conception and design of the study, data acquisition, and drafting and revision of the manuscript. VB contributed substantially to the design of the study, review of data analysis and interpretation, and drafting and revision of the manuscript. AS contributed substantially to the conception and design of the study, data analysis, and drafting and revision of the manuscript. MBD contributed substantially to the conception and design of the study, data acquisition, data analysis, and drafting and revision of the manuscript. MBD takes sole responsibility for data analyses. All authors (RAP, AGH, VB, AS, and MBD) approved the final version of the manuscript.
Data Sharing: The data are not publicly available due to the small study size and single-center setting, which could compromise the privacy of the research participants. Data requests should be sent to author: MBD. Restrictions may apply to the availability of these data, which were generated under an agreement with the funder. Data requests will need to align with university and funder policies prior to release and will be accepted only with the permission of the funder.
Writing, editorial support, and formatting assistance for this manuscript were provided by Nicola Welch, PhD, CMPP, for Cactus Life Sciences (part of Cactus Communications), which was contracted and compensated by BIPI for these services.
Declaration of Interest
The authors received no direct compensation related to the development of this manuscript. RAP reports research grants from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., (BIPI), GlaxoSmithKline, and Teva. AGH has no competing interests to disclose. AS and VB are employees of BIPI. MBD reports grants from BIPI to conduct this study and provides consulting services for BIPI. MBD also reports research grants from the National Institutes of Health, the Department of Defense, the Patient-Centered Outcomes Research Institute, the American Lung Association, Midmark Corporation, and Teva unrelated to this work and MBD reports personal consulting fees from BIPI, GlaxoSmithKline, AstraZeneca, Teva, Midmark, Verona, Chiesi, and Polarean, Inc., unrelated to this work.