Running Head: Dietary Fiber Intake and Prevalence of COPD
Funding Support: None
Date of Acceptance: February 5, 2024 | Publication Online Date: February 14, 2024
Abbreviations: BMI=body mass index; CBC=complete blood cell; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRP=C-reactive protein: DF=dietary fiber; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; IL=interleukin; LPS=lipopolysaccharide; MEC=Mobile Examination Center; NCHS=National Center for Health Statistics; NHANES=National Health and Nutrition Examination Survey; OR=odds ratio; Q1=first quartile; Q2=second quartile; Q3=third quartile; Q4=fourth quartile; RCS=restricted cubic spline; SCFAs=short-chain fatty acids; SD=standard deviation; TNF-α=tumor necrosis factor-alpha; WBC=white blood cell
Citation: Jin J, Bian Y, Gu Z, Lin M. Association between dietary fiber intake and prevalence of chronic obstructive pulmonary disease in a middle-aged and elderly population: a study based on the National Health and Nutrition Examination Survey database. Chronic Obstr Pulm Dis. 2024; 11(2): 216-228. doi: http://doi.org/10.15326/jcopdf.2023.0457
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease,1 characterized by inflammation and remodeling of the lower respiratory tract and lung parenchyma, as well as the activation of inflammatory and immune processes.2 COPD is progressive and not only manifests in the lungs but is also associated with disease development in other parts of the body, including increased risks of cardiovascular diseases, osteoporosis, and depression, severely impacting people's physical health.3,4 In 2019, there were approximately 212.3 million COPD cases globally, leading to 3.3 million deaths,5 making it the third primary cause of death globally after coronary heart disease and stroke.6 COPD is more prevalent in the middle-aged and elderly population, with a prevalence rate of up to 13.7% in individuals over the age of 40. COPD affects a large population, and unfortunately, even with optimal treatment, patients experience periodic exacerbations leading to a decrease in lung function and quality of life, elevated death risk, and higher treatment costs.7 Currently, available drug treatments for COPD have shown limited effectiveness, emphasizing the importance of identifying effective preventive and therapeutic factors to lower prevalence of COPD.
Smoking is a key environmental risk factor for COPD. Nevertheless, despite sharing a similar smoking history, not everyone gets COPD. Therefore, genetic susceptibility, environmental pollution,8 and even dietary9 factors are believed to affect the prevalence of COPD. A high dietary fiber (DF) intake lowers lung inflammation and risk of developing COPD through its antioxidant and anti-inflammatory properties.10 DF increases concentration of short-chain fatty acids (SCFAs) by altering composition of the gut microbiota.11 These byproducts exist in the systemic circulation and can protect lung function and prevent COPD by regulating macrophages, neutrophils,12 and alleviating pulmonary inflammation.13 Several prospective studies14-16 have reported the association between DF intake and the prevalence of COPD. A large-scale prospective study conducted in the United States indicated that, after adjusting for age, gender, and other confounding factors in a multivariate model, the risk of newly diagnosed COPD decreases with an increase in total fiber intake.16 Moreover, similar results are observed in gender-specific analyses. A study involving a large cohort of men (45,058 participants) found a robust negative correlation between fiber intake and COPD among current smokers/ex-smokers.14 Another cohort study focusing on women also found a negative correlation between high DF intake and the risk of COPD. The study explored the negative correlation between total DF intake and the risk of COPD in patients with different smoking statuses (current, former smokers, never smokers).15 However, the above studies only examined the relationship between DF and COPD prevalence and did not delve into the potential mediating effect of DF intake on COPD prevalence.
Therefore, based on previous studies, we utilized data from 3 cycles (2007–2012) of the National Health and Nutrition Examination Survey (NHANES) to investigate the relationship between DF intake and COPD prevalence. Furthermore, we conducted a detailed analysis of the relationships among DF intake, white blood cell (WBC) count, and COPD prevalence. Understanding the importance of dietary factors in respiratory health can provide insights for future public health interventions aimed at preventing COPD in the middle-aged and elderly population.
Methods
Data Source and Study Population
The NHANES database, conducted by the National Center for Health Statistics (NCHS) in the United States, was used as the data source.17 NHANES is a stratified, multistage study that combines interviews, physical examinations, and laboratory tests to assess the health and nutritional status of the U.S. population. The database is freely accessible and has been approved by the NCHS Institutional Review Board, with informed consent obtained from the participants.
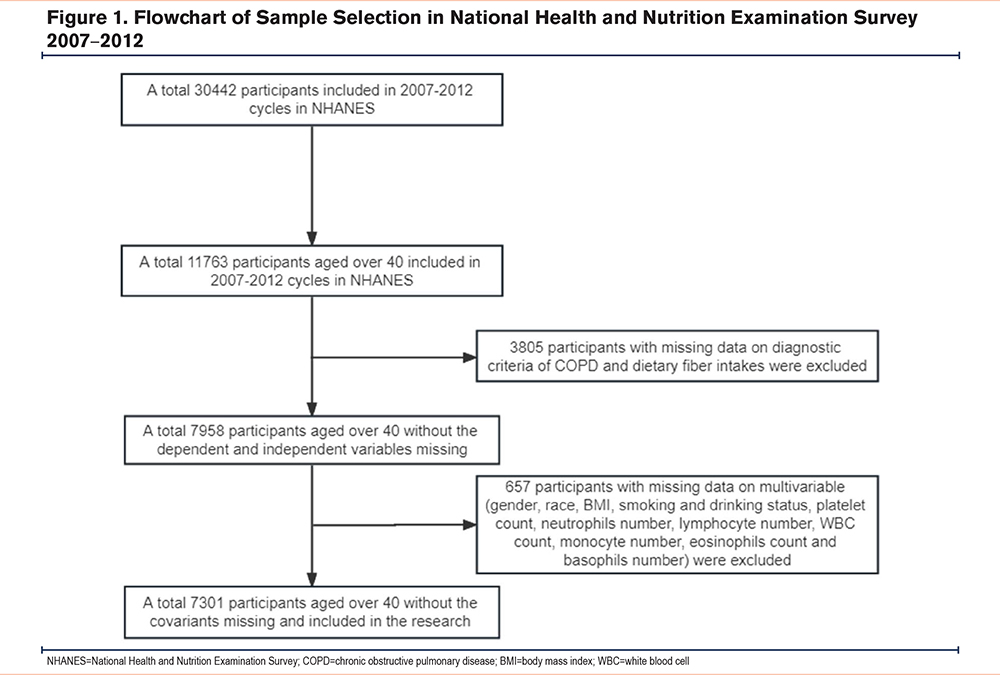
This study selected information from 30,442 respondents across 3 consecutive cycles (2007–2012) in the database. The exposure variable in the research was DF intake, and the outcome variable was the occurrence of COPD. The study initially involved data collection from 11,763 respondents aged 40 and above. Exclusions were made for 3805 individuals lacking COPD diagnostic or DF intake information, as well as 657 cases with missing covariates (gender, race, body mass index [BMI], smoking and drinking status, platelet count, neutrophil count, lymphocyte count, WBC count, monocyte count, eosinophil count, and basophil count). Ultimately, 7301 respondents with complete and qualifying information were included in the study. The detailed participant selection process is illustrated in Figure 1.
Dietary Fiber Intake
Data collection for DF intake was based on a 24-hour dietary recall questionnaire in the NHANES database. Dietary recall of the participants was conducted twice: first during a Mobile Examination Center (MEC) visit and then through a telephone follow-up 3 to 10 days later. The DF intake of the participants was evaluated based on the U.S. Department of Agriculture’s Food and Nutrient Database for Dietary Studies.18 The data from both recalls were averaged for participants with no missing data, and the DF intake was log-transformed to approximate a normal distribution.19
COPD
In this study, COPD was confirmed through a medical conditions questionnaire and the forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio after bronchodilator inhalation. In the NHANES data collection questionnaire, the presence of COPD was determined if the respondent answered "yes" to either of the 2 questions: "Have you ever been told that you have chronic bronchitis?" or "Have you ever been told that you have emphysema?" Additionally, a diagnosis of COPD was made if the FEV1/FVC ratio after bronchodilator inhalation,1,20 was less than 0.70.
Covariates
Gender, age, race, BMI, smoking and alcohol consumption status, platelet count, neutrophil count, eosinophil count, lymphocyte count, WBC count, monocyte count, and basophil count were identified as potential confounding factors in this study. COPD is a major public health issue in individuals aged 40 and above; therefore, this study only included participants aged 40 and above.1,21 The age of the participants was categorized as middle-aged (40–59 years) and older adults (≥60 years). Male and female were used to categorize gender. Race was categorized as Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other races. BMI was classified as ≤25 kg/m2, 25–30 kg/m2, or >30 kg/m2. Smoking status was categorized as "now smoking," "former smoking," or "never smoked."22 Alcohol consumption was determined by the response to the question "Had at least 12 alcohol drinks/1 year?" with options of "yes" or "no" (one drink refers to 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of distilled spirits).23 Complete blood cell (CBC) count with WBC differential was tested by the volume-conductivity-scatter method. The Beckman Coulter MAXM instrument at the MEC provided CBC information and blood cell distribution for the participants.24 The collected CBC information included platelet count, WBC count, lymphocyte count, neutrophil count, basophil count, monocyte count, and eosinophil count.
Statistical Analysis
In this study, the tableone package was used to generate baseline tables. The sample size and proportions of categorical variables, as well as mean and standard deviation (SD) of continuous variables (unweighted n; n(%), mean, and SD adjusted for weights), were calculated and presented per the presence or absence of COPD. The survey package was utilized to construct weighted logistic regression models for the association of DF intake with COPD in the middle-aged and older adult population. Separate weighted logistic regression models were developed for continuous and categorical variables in the unadjusted model, and stratified analysis was performed for categorical variables. DF intake was stratified into quartiles, and a weighted logistic regression model adjusted for confounding factors was constructed using the survey package to assess the association of DF intake with COPD, incorporating stratified analysis and baseline table discrepancies for gender subgroup analysis. Restricted cubic splines (RCSs) were employed in the unadjusted and adjusted weighted logistic regression models to dissect the association of DF intake with COPD. The mediation package was utilized to examine the possible mediating effect of DF intake on COPD, with bootstrap testing conducted to estimate mediation proportions and corresponding 95% confidence intervals (CIs) for each of the 1000 bootstrapped samples in the unadjusted model. The models in this study included: Crude (unadjusted), model I (adjusted for gender, age, and race), model II (adjusted for gender, age, race, BMI, smoking, and alcohol consumption status), and model III (adjusted for gender, age, race, BMI, smoking, alcohol consumption status, platelet count, and WBC count). R (V4.2.1, the R Foundation) was used for all statistical calculations, and a 2-sided p-value <0.05 was regarded as statistically significant.
Results
Baseline Characteristics of Participants
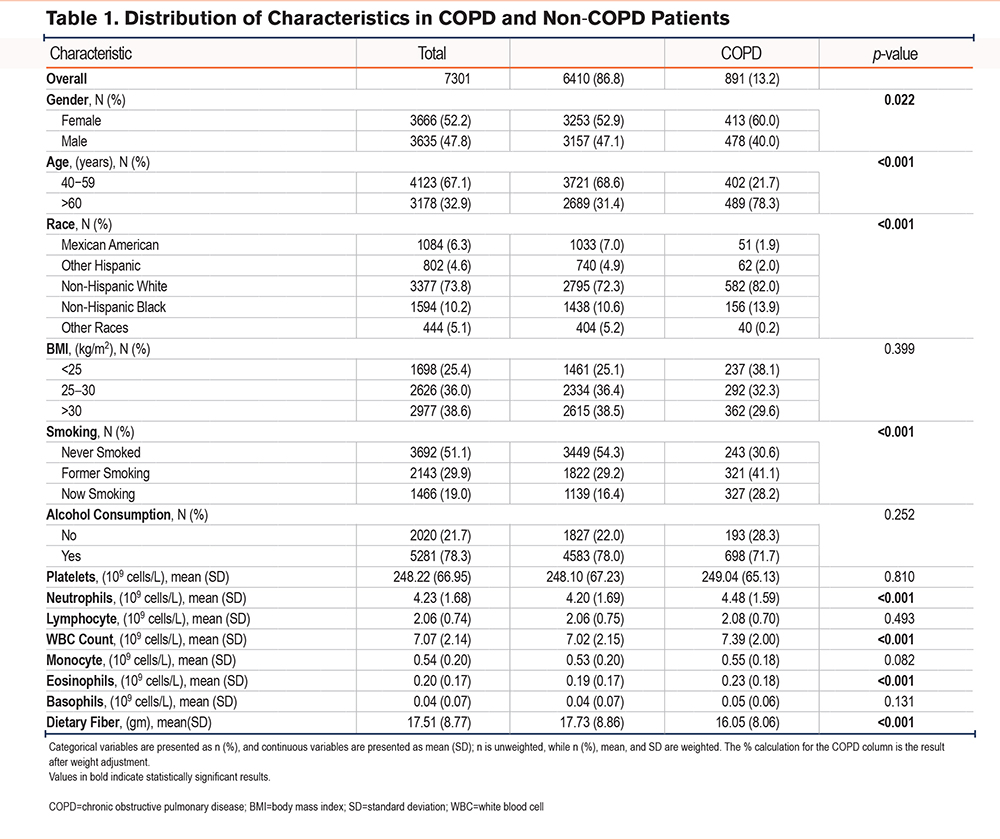
The distribution of baseline characteristics among 7301 participants aged 40 years and above is presented in Table 1. The proportion of males (47.8%) was lower than females (52.2%), with middle-aged (40–59 years) and elderly (≥60 years) individuals accounting for 67.1% and 32.9%, respectively. The prevalence of COPD among participants was 13.2%. Clinical characteristics of the participants stratified by the presence of COPD revealed significant statistical differences (p<0.05) in gender, race, age, smoking status, neutrophil count, WBC count, and eosinophil count among groups. The COPD patient population was predominantly elderly individuals (78.3%) and non-Hispanic White (82.0%). Baseline results showed that the mean DF intake of COPD patients (16.05 ± 8.06) was significantly lower than that of non-COPD patients (17.73 ± 8.86) (p<0.001).
Association of Complete Blood Cell/Dietary Fiber Intake With COPD
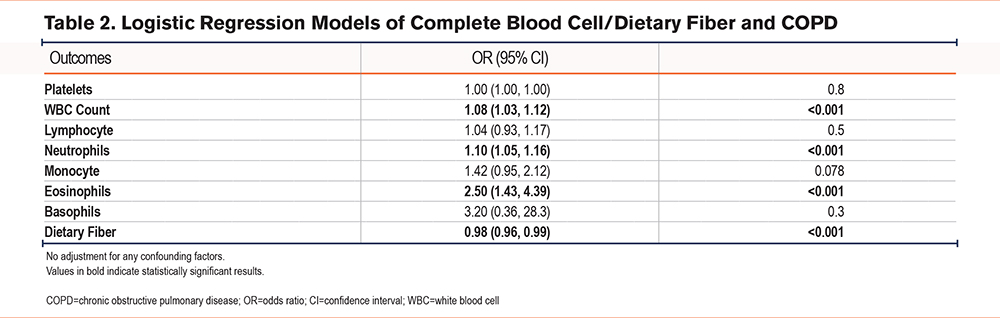
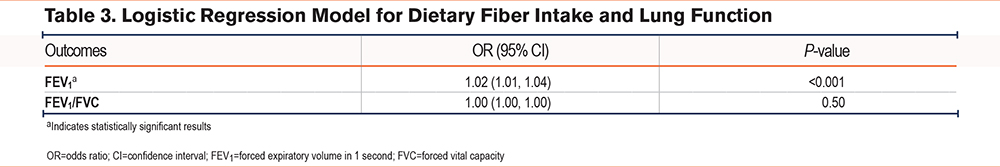
A weighted logistic regression model was employed to assess the association of DF intake with COPD. Results without adjusting for any confounding factors are presented in Table 2. According to the data, there was a significant positive correlation between WBC count (odds ratio [OR]: 1.08, 95% CI: 1.03–1.12, p<0.001) and the prevalence of COPD. Additionally, an increase in DF intake was associated with a significant reduction in the risk of COPD (OR: 0.98, 95% CI: 0.96–0.99, p<0.001). Furthermore, we explored the relationship between DF intake and lung function (indicating the severity of COPD), as shown in Table 3. We found a positive correlation between DF intake and FEV1 (OR: 1.02, 95% CI: 1.01–1.04, p<0.001), with no statistical significance in FEV1/FVC (p>0.05).
Stratified Analysis
Stratified analysis of the weighted logistic regression model for DF intake and COPD was conducted based on gender, race, age, smoking status, BMI, and alcohol consumption, as shown in Table 4. Among males, middle-aged individuals (40–59 years), individuals with BMI≤30 kg/m2, those with a history of smoking, and drinkers, an increased DF intake was significantly linked to reduced prevalence of COPD (p<0.05).
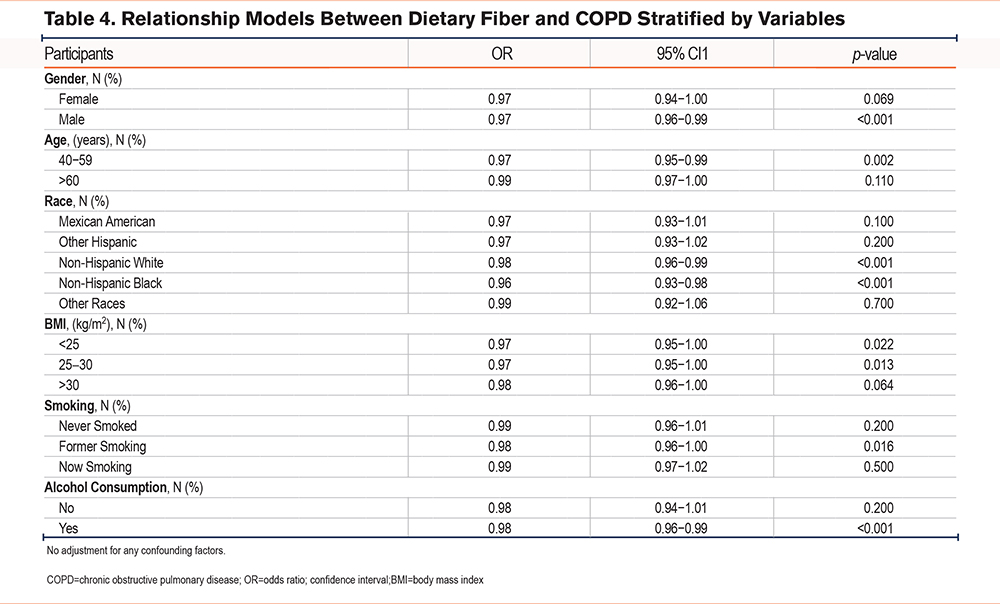
Subgroup Analysis
As presented in Table 5, in unstratified population, the Crude model (OR: 0.98, 95% CI: 0.96–0.99, p<0.001), model I (OR: 0.97, 95% CI: 0.96–0.99, p<0.001), model II (OR: 0.98, 95% CI: 0.97–1.00, p<0.05), and model III (OR: 0.98, 95% CI: 0.97–1.00, p<0.05) all indicated a significant reduction in the prevalence of COPD with increased DF intake. Furthermore, the impact of DF intake levels on the prevalence of COPD was investigated and compared to the first quartile (Q1) of DF intake, and any quartile in the 4 models significantly reduced the risk of COPD (p<0.05). Moreover, there was a trend in the prevalence of COPD with changes in quartile intervals of DF intake (p<0.05). Furthermore, when considering the overall population, participants in the second to fourth quartiles (Q2–Q4) of DF intake exhibited an approximately 35% lower probability of developing COPD compared to those in Q1. Subgroup analysis based on gender (Table 6) showed that in males, the Crude model (OR: 0.97, 95% CI: 0.96–0.99, p<0.001), model I (OR: 0.97, 95% CI: 0.96–0.99, p<0.001), model II (OR: 0.98, 95% CI: 0.97–1.00, p<0.05), and model III (OR: 0.98, 95% CI: 0.97–1.00, p<0.05) all showed a significant decrease in the prevalence of COPD with increased DF intake, while no significant relation was seen in the female population.
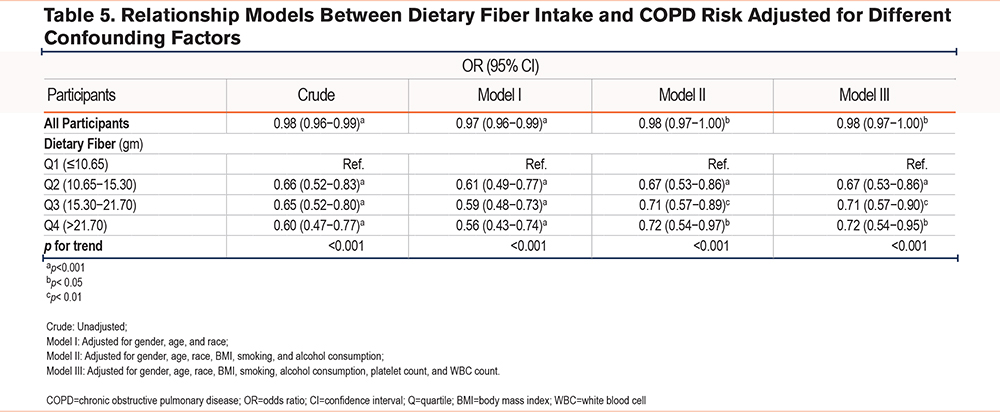
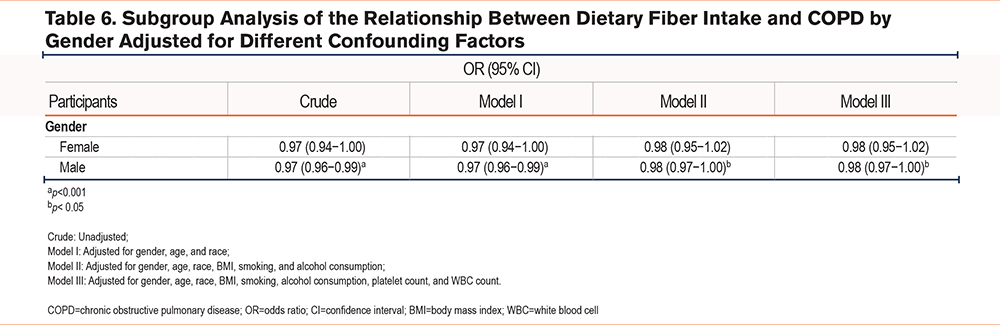
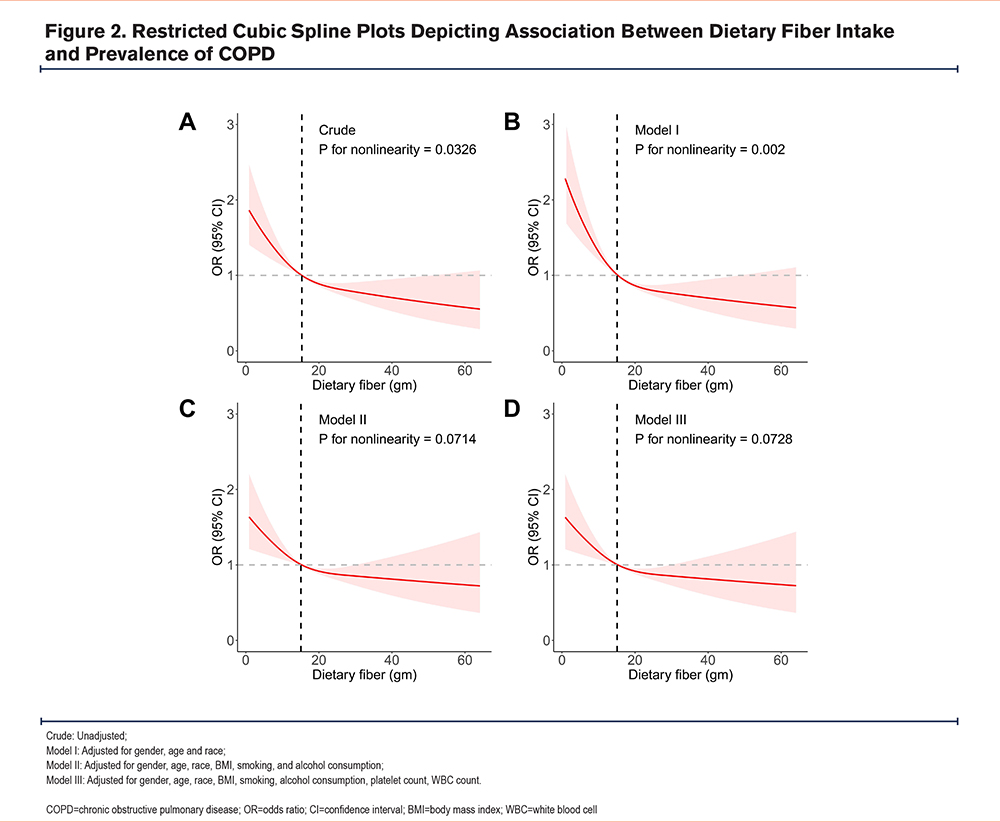
Nonlinear Association of Dietary Fiber Intake With Prevalence of COPD
Figure 2 depicts the dose-response relationship between DF intake and the prevalence of COPD. Results from RCS curves indicated a nonlinear association detected in both the Crude model (p for nonlinearity =0.0326) and Model I (p for nonlinearity =0.002). However, this nonlinear association disappeared in Model II and Model III, which were further adjusted for confounding factors (p for nonlinearity >0.05). Across all 4 models in the RCS curves, there was a consistent negative correlation between DF intake and the prevalence of COPD, suggesting a stable association between the 2. Additionally, based on the data, the critical threshold for the impact of DF intake on COPD prevalence was 15.10 gm. When DF intake exceeded 15.10 gm, it could effectively lower the prevalence of COPD.
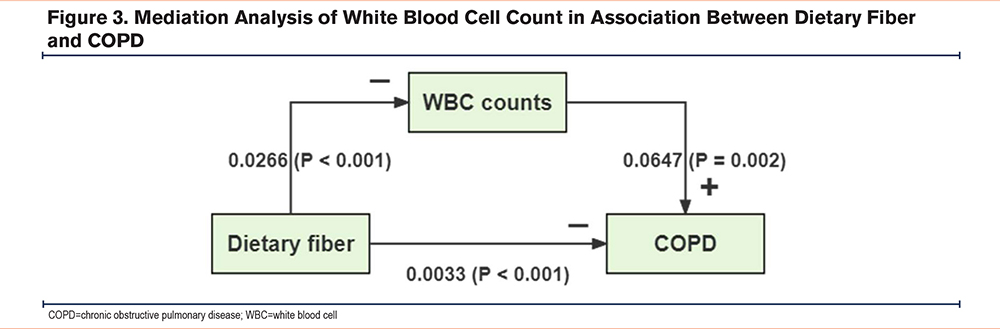
Mediation Analysis
As depicted in Figure 3, the unadjusted model indicated a significant effect of DF intake on COPD (p<0.001). The mediation effect generated through WBC count was also highly significant in relation to the prevalence of COPD (p=0.002), indicating that WBC counts partially mediated the association of DF intake with COPD, with a mediation proportion of 9.89% (p=0.006). Therefore, we believe that the increase in DF intake may lower the prevalence of COPD by regulating WBC count.
Discussion
In this study, we used data from the nationally representative NHANES (2007–2012) to assess the association between DF intake and the prevalence of COPD. The results suggested that DF intake was associated with a reduction in the prevalence of COPD, especially in middle-aged and elderly men, and may be related to the severity of COPD. Mediation analysis showed that increased DF intake may reduce the prevalence of COPD by regulating WBC count.
The pathophysiology of COPD involves various inflammatory cell types, including macrophages,25 neutrophils,26 and T cells,27 which coordinate and sustain the inflammatory response in the lungs in response to toxic gases. In addition to pulmonary reactions, chronic systemic low-grade inflammation is also commonly observed in COPD patients.28,29 Studies have shown that acute-phase proteins such as C-reactive protein (CRP), fibrinogen, and various proinflammatory cytokines such as interleukin-1β (IL-1β),30 chemokines,31 and tumor necrosis factor-alpha (TNF-α)32 increase in COPD patients, especially as the disease progresses, and the complications caused by these factors are main causes of death in COPD patients.33
DF is an important component of a healthy diet, and increasing evidence suggests its significant role in various chronic diseases.34,35 In a previous cross-sectional study, participants with the highest intake of DF had a 63% lower risk of increased CRP concentrations than those with the lowest total fiber intake.36 This finding was confirmed in another survey study.37 Earlier studies have suggested a positive correlation between a “Western” diet (high in refined grains, red and processed meats, fried potatoes, eggs, and soft drinks) with low fiber content and COPD risk,38 while a “prudent” dietary featured by high fiber intake (abundant in vegetables, fruits, whole grain foods, and fish) was negatively associated with COPD prevalence.39 The results of this study indicated that participants in the Q2−Q4 DF intake quartiles had an approximately 35% lower probability of developing COPD than those in Q1. This finding is consistent with previous research, highlighting the significant role of increased DF intake in reducing the prevalence of COPD. Furthermore, our study revealed a correlation between DF intake and the severity of COPD, showing a positive association with FEV1. This aligns with existing literature, which links low DF intake to decreased lung function indicators and increased rates of airflow limitation in participants.40 It is also consistent with the severity of airway damage in elderly male COPD patients.41 On the contrary, higher DF intake is associated with improved lung function and reduced prevalence of COPD.42 These data further underscore the potential role of a diet rich in fiber-rich foods in enhancing pulmonary health.
In this study, the difference in fiber intake between participants with and without COPD was approximately 1.7 g/day. Simultaneously, the results of the RCS curve analysis indicated that the critical threshold for the impact of DF intake on COPD prevalence was 15.10 g. When DF intake exceeded 15.10 g, it could effectively lower the prevalence of COPD, while lower intake may potentially increase the risk of COPD occurrence. Additionally, based on findings from related studies, each gram increase in total fiber intake (up to 25 g/day) is associated with a 3% reduction in COPD risk (95% CI 2%–5%).15 The data in this study indicated that individuals with COPD (16.05 g/day) and those without COPD (17.73 g/day) had DF intake within a similar range. We reasonably speculated that there might be a critical threshold for DF intake within this range that contributed to the occurrence of COPD. This further suggests that increasing DF intake may have a significant impact on reducing the prevalence of COPD.
We speculated that the potential mechanism by which DF protects against COPD may be related to its anti-inflammatory effects in regulating systemic inflammation. DF can influence the levels of chronic inflammation by altering intestinal pH, reducing membrane permeability, and activating G protein-coupled receptors (GPCRs). SCFAs from the fermentation of DF play a crucial role in the anti-inflammatory process.43 DF is the main source of SCFAs in the intestines, with acetate, propionate, and butyrate being the highest in concentration in the human body.44 Numerous studies have shown that SCFAs decrease the production of pro-inflammatory mediators and increase the release of anti-inflammatory mediators, thus mitigating inflammation. For example, butyrate can inhibit the production of pro-inflammatory mediators in lipopolysaccharide (LPS) and cytokines, including TNF-α, IL-6,45 and nitric oxide,46 while increasing the release of anti-inflammatory cytokine47 IL-10. An animal experimental study demonstrated that oral administration of butyrate effectively lowers concentrations of TNF-α, IL-1β, and nitric oxide in bronchoalveolar lavage fluid and decreases alveolar hemorrhage and neutrophil infiltration in mice with acute lung injury induced by tracheal instillation of LPS.48 Other studies have indicated that butyrate and propionate inhibit TNF-α secretion and nuclear factor kappa-light-chain-enhancer of activated B cell activity, suppress expression of the anti-inflammatory cytokine IL-10 in LPS-activated monocytes and neutrophils through activation of GPCRs and suppression of histone deacetylase .49-52 Acetate can inhibit LPS-induced TNF-α and IL-6 secretion in human monocytes by activating free fatty acid receptors.53
Furthermore, the association of DF intake with COPD was controversial when considering gender differences. Previous studies presented a significant negative correlation between total DF intake and COPD in female populations (relative risk 0.62, 95% CI: 0.46–0.85; p<0.01).16 In contrast, in our study, the relationship between the 2 variables was not significant in the female population (OR: 0.97, 95% CI: 0.94–1.00; p=0.069). However, a study conducted on a male population (hazard ratio =0.62; 95% CI: 0.50–0.78, p<0.0001) showed results congruous with our study trend (OR: 0.97, 95% CI: 0.96–0.99; p<0.001).14 In the results mentioned above, it is evident that the association of DF intake with the prevalence of COPD differs by gender. This difference may be attributed to factors such as tobacco use rates and variations in DF intake among different populations. Studies have found a significant protective impact of DF against COPD in populations with a history of smoking, but no such association was observed in never-smokers,14,15 and the prevalence of tobacco use is higher in males than females.54 Additionally, research conducted on the elderly population has found that inadequate DF intake is more common in males.55 Therefore, ensuring an adequate DF intake is particularly effective in reducing prevalence of COPD in middle-aged males. However, the mechanisms underlying the gender differences are still unclear and require further detailed experiments for exploration.
COPD is a chronic disease that progresses to systemic inflammation. As inflammation continues, the concentration of pro-inflammatory cytokines in lung tissues and systemic serum gradually increases, along with enhanced oxidative stress. Activated WBCs and inflammatory markers such as TNF-α are significantly elevated in COPD patients.56 The primary cause of chronic inflammation in COPD is the recruitment of WBC subsets, neutrophils, and lymphocytes. Once activated, neutrophils release elastase, tissue proteases, matrix metalloproteinases, and myeloperoxidase, which actively participate in pathological mechanisms of emphysema and COPD, leading to the destruction of lung tissues in COPD patients.57 Current research has focused on potential neutrophilic inflammation in COPD, and studies58 have demonstrated a significant relationship between the 2. Additionally, eosinophils have been proposed as a personalized clinical biomarker for reducing inhaled corticosteroid treatment in COPD patients.8 Eosinophils, composed of bilobed nuclei and large cytoplasmic granules, are inflammatory WBCs that can be recruited to the lungs under certain conditions to participate in the inflammatory response in COPD.59 They have the capacity to synthesize and release chemokines (such as CCL5, CCL11, CCL13), growth factors (transforming growth factor ), cytokines (IL-2, IL-3, etc.),60-62 and cytotoxic granule proteins (mainly basic proteins, eosinophil peroxidase, eosinophil cationic protein, and eosinophil-derived neurotoxin),63 which exert pro-inflammatory effects. Our results displayed a significant positive correlation between neutrophil count, WBC count, eosinophil count, and the prevalence of COPD. Consequently, WBC count plays an extremely pivotal role in COPD diagnosis and treatment, and increased WBC count is considered an independent criterion for antibiotic treatment in exacerbated COPD patients in most clinical guidelines.64
In the stratified analysis of the relationship model between DF intake and the prevalence of COPD after adjusting for confounding factors in this study, an issue arises where the effect sizes appear similar, but the confidence intervals are wide. This could be attributed to small sample sizes in certain subgroups, potentially leading to insufficient statistical power and influencing the significance of some effects. Moreover, based on the data from the model, the impact of DF on COPD prevalence in different population strata shows relatively minor variations, indicating a need for exploration within the broader cohort of the overall population. Considering the generality of our study results in the entire population and the model constructed after adjusting for numerous confounding factors related to COPD, as well as the exploration through intermediate analysis of the associations among DF intake, WBC count, and COPD, this study provides valuable guidance for our future research endeavors.
However, the study has limitations. First, being a cross-sectional study, it cannot establish a causal relationship between DF intake and COPD. Second, the calculation of DF intake relies on data obtained from dietary questionnaire surveys, which may be subject to memory bias. Additionally, the DF intake of COPD patients may be restricted due to the impact of the disease (e.g., patients with severe airway damage may find food preparation and consumption more challenging due to respiratory difficulties and fatigue). Moreover, during the follow-up period, COPD diagnosis was based on postbronchodilator values and a fixed FEV1/FVC ratio, as well as self-reporting. However, the study cannot entirely rule out the possibility of misdiagnosis leading to underdiagnosis or overdiagnosis of COPD in some participants. Finally, COPD is influenced by numerous factors, and despite including as many relevant covariates as possible in the model, the study cannot eliminate the impact of unmeasured or residual covariates. Despite these limitations, our research demonstrates a negative correlation between increased DF intake and COPD prevalence.
Conclusions
Through an in-depth analysis of 3 consecutive cycles of nationally representative NHANES data from 2007 to 2012, we have explored the relationship between DF intake and the prevalence of COPD. The results indicated a significant reduction in the prevalence of COPD with an increase in DF intake. Stratified analysis revealed that the increased intake of DF significantly reduced the risk of COPD in populations with a history of smoking, with a history of alcohol consumption, and of middle-aged and elderly individuals, as well as those with a BMI ≤ 30 kg/m². RCS analysis explored the dose-response relationship between DF and the prevalence of COPD, suggesting an effective reduction in COPD prevalence when DF intake >15.10 grams. Mediation analysis delved into the association between DF intake and COPD, suggesting that the increased intake of DF may reduce the risk of COPD by modulating WBC count. This study provided new insights into understanding the relationship between DF and COPD. Furthermore, through mediation analysis of WBC count, we revealed that DF may influence the development of COPD by regulating systemic inflammation levels. This offers a crucial foundation for future in-depth research and provides valuable insights for developing dietary strategies beneficial in preventing COPD.
Acknowledgements
Author contributions: JJ was responsible for the conception and design of the manuscript. YB was in charge of administrative support. ZG was responsible for the provision of study materials and patients. The collection and assembly of the data was done by JJ and ML. Data analysis and interpretation was provided by YB. The manuscript writing was done by JJ and ZG. All authors gave their final approval of the manuscript.
Data Sharing: The data in this study are available from the corresponding author on reasonable request.
Declaration of Interests
The authors declare that they have no conflicts of interest.