Running Head: CAT Score Progression Link to Exacerbation Risk
Funding Support: This study was funded by GSK (Study 212648/HO-19-19947). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit for publication.
Date of Acceptance: February 23, 2024 | Publication Online Date: February 28, 2024
Abbreviations: %pred=percentage predicted; ACOS=asthma-COPD overlap syndrome; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; ED=emergency department; EXACT=Exacerbation of Chronic Pulmonary Disease Tool; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; HR=hazard ratio; ICS=inhaled corticosteroid; IQR=interquartile range; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; mMRC=modified Medical Research Council; PRO=patient-reported outcome; SD=standard deviation
Citation: Jones P, Soutome T, Matsuki T, et al. Health status progression measured using weekly telemonitoring of COPD Assessment Test scores over 1 year and its association with COPD exacerbations. Chronic Obstr Pulm Dis. 2024; 11(2): 144-154. doi: http://doi.org/10.15326/jcopdf.2023.0415
Online Supplemental Material: Read Online Supplemental Material (861KB)
Note: Data included in this manuscript was previously presented at the 2022 European Respiratory Society International Congress.
Introduction
The use of diary cards administered via paper or electronically to measure patient-reported outcomes (PROs) in chronic obstructive pulmonary disease (COPD) has been validated,1 though patients often prefer an electronic format.2 The COPD Assessment Test (CAT) was developed to provide a quick, easy-to-use tool to assess health status that is applicable worldwide.3 Its reliability and validity are established,4 and the Global initiative for chronic Obstructive Lung Disease (GOLD) strategy report recommends it as a comprehensive health status questionnaire developed to be applicable worldwide.5 Its main advantages are coverage of important clinical aspects of COPD and a short time required for completion.6 Along with the Exacerbation of Chronic Pulmonary Disease Tool (EXACT) exacerbation diary, the CAT was found to be more responsive than other measures during recovery from a severe exacerbation.7 In this context, it should be noted that the EXACT is a daily diary specifically designed to detect and quantify episodic events like exacerbations.8,9 Whereas the CAT is a disease-specific health status measure designed to quantify impairment of health status due to any cause.3
Increasing CAT scores are associated with worsening health status. Among patients with a history of exacerbations, CAT scores predict the risk of further exacerbations in the following 6 months.10 The link between exacerbations and the evolution of CAT scores over time was further demonstrated in a study in Switzerland, which found a positive association between the evolution of CAT over time and incidence of exacerbations.11 Change over time in CAT score may also provide a responsive outcome measure in clinical studies. In a recent trial, patients randomized to telemedicine showed a lower rate of worsening in CAT scores compared with those receiving standard care.12
While the use of weekly CAT has been reported previously, replication of these findings in Japan is needed, as Japanese patients typically report low scores on the CAT and St George’s Respiratory Questionnaire,13 and reporting of exacerbations is less frequent in Japan compared with the rest of the world.14,15 The feasibility and acceptability of using telemedicine to support standard care of patients with COPD or asthma-COPD overlap syndrome (ACOS) in Japan has recently been explored.16
This study used the same platform and aimed to assess the evolution of CAT scores over time and its relationship with exacerbations among patients with COPD.
Methods
Study Design
Full details of the study design, patient population, telemedicine platform, and preliminary data analysis have been published elsewhere.16 The study was conducted in accordance with applicable local regulations, and the principles stated in the Declaration of Helsinki. All study documents were reviewed and approved by institutional review boards and/or independent ethics committee(s) at all investigational sites.16 All participants provided written, informed consent. YaDoc data was extracted and deidentified in accordance with the Personal Information Protection Act of Japan. Briefly, this was a 52-week multicenter, prospective, single-arm, cohort study assessing the feasibility and acceptability of the use of a telemedicine platform (GSK study: jRCT1080224832) for COPD management. In this study, we used the same platform to examine changes in weekly CAT scores.
Patient Population
Japanese participants aged 40 years or older with an established clinical history of COPD or ACOS, according to the American Thoracic Society/European Respiratory Society17 and Japanese Respiratory Society COPD and ACOS18 guidelines, and who could provide informed consent and participate were enrolled in the study. Participants were selected from 6 sites where the telemedicine YaDoc platform (Integrity Healthcare Co. Ltd, Tokyo, Japan) was implemented.16 Participants must have undergone lung function testing within the previous 12 months and be receiving maintenance inhaled therapy with a long-acting muscarinic antagonist (LAMA) and/or long-acting beta2-agonist (LABA). LAMA/LABA or inhaled corticosteroid (ICS) combinations were permitted but use of triple therapy (ICS/LAMA/LABA) or biological therapy was not. Participants receiving triple therapy were excluded with the aim of recruiting a study population that was typical of patients with COPD in Japan, rather than restricting participation to specific subgroups of patients such as those with frequent exacerbations, or those with ACOS, who may not be typical of the broader COPD population in this country. Full inclusion and exclusion criteria are presented in Supplementary Table 1 in the online supplement.
Outcomes
The primary objective of this analysis was to evaluate the evolution of CAT scores over time. The secondary objective was to assess the relationship between longitudinal changes in CAT and exacerbations of COPD and was hypothesis-generating. Exacerbations were identified via health care records and included those identified by a physician as a COPD exacerbation, those requiring courses of systemic steroids or antibiotics, or requiring hospitalization or a emergency department (ED) visit. Evolution of the CAT score over 52 weeks was assessed for the total population and, in a post hoc analysis, for a subgroup of participants who completed at least 25% of the 52 weekly CAT entries. Completion below this threshold was considered not sufficient to gain a reliable insight into health status. The proportion of participants with CAT score changes of more than 4 units (i.e., at least twice the minimum clinically important difference) over the look-back periods of 4 and 8 weeks was investigated, as well as the association between the evolution of the CAT score and COPD exacerbations. An analysis of the initial learning effect on CAT scores was also performed.
Data Collection and Analysis
Participants were trained to use the telemedicine platform YaDoc at study entry (new user) or at initiation (existing user) by a physician or facility staff member. Participants used the YaDoc smartphone application to complete electronic PROs, including weekly questions from the CAT.19
To evaluate change in the CAT score over 52 weeks, eachparticipant was categorized into 1 of 3 groups using linear regression of change over time: (1) improved (negative slope with upper limit of 95% confidence interval [CI] of slope estimate below 0); (2) worsened (positive slope with lower limit of 95% CI of slope estimate above 0); and (3) stable health status (all other cases) based on CAT change over time. This evaluation was performed for the total population and, in a post hoc analysis, for a subgroup of participants who achieved at least 25% compliance in completion of the CAT.
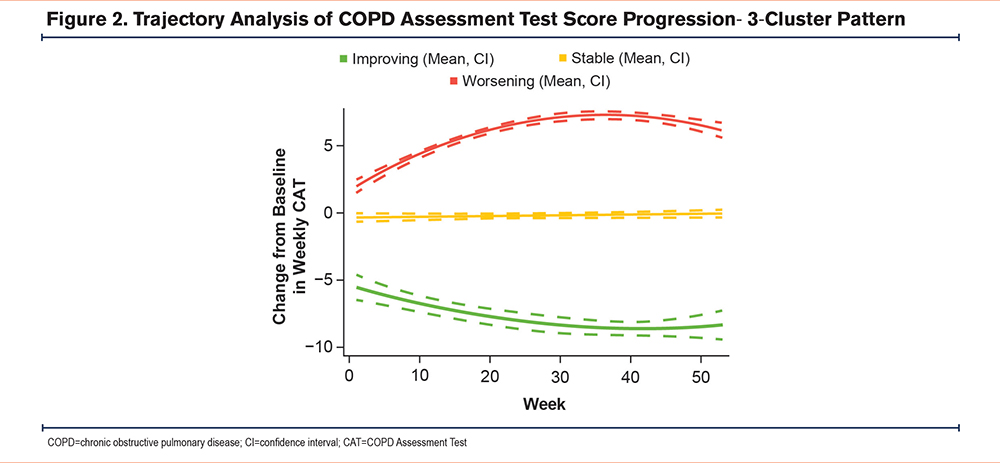
Group-based trajectory modeling was used to identify clusters of individuals who followed similar patterns of evolution of the CAT during the study period,20 and clusters were characterized by patient demographics. This analysis was performed based on changes from baseline in weekly CAT score (using median changes for participants with multiple records in a single analysis week) and the analysis week used for convergence. Group trajectories were estimated assuming the distribution of the evolution of CAT scores followed a censored normal distribution, with upper and lower censoring points of 40 and -40, respectively. The optimal number of clusters (2, 3, or 4) was determined based on Bayesian Information Criteria.
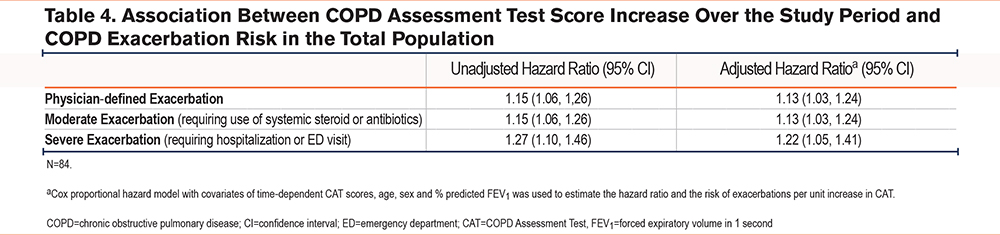
The association between the evolution of the CAT score and COPD exacerbations wasevaluated using the Andersen–Gill formulation of the Cox proportional hazards model with covariates of time-dependent CAT scores, age, sex, and percentage predicted (%pred) forced expiratory volume in 1 second (FEV1) for moderate exacerbations (requiring corticosteroids and/or antibiotics) and severe exacerbations (requiring hospitalization or an ED visit).5 Moderate and severe exacerbations were assessed separately as a sensitivity analysis. No formal analyses assessing differences between groups based on combined CAT score and exacerbation profile were performed.
A test for the presence of an initial learning effect for completion of the CAT was performed using a second-order regression model in comparison with a linear model. A learning effect was assumed if the second-order model produced a significant improvement in goodness of fit compared with linear regression. This method of comparing goodness of fit across models has been used to assess a learning effect in COPD-focused studies.11
Results
Study Population
Overall, 72 of the 84 (86%) participants enrolled completed the study,16 and the median weekly CAT completion rate was 83%. Baseline demographics and clinical characteristics are shown in Table 1 and have been published previously.16 Most participants were male (n=74; 88%) and the mean (standard deviation [SD]) age was 68.7 (9.17) years. Approximately one-fifth (22.6%) of participants were current smokers, with the remainder being former smokers. The population was considered representative of a population with mild COPD: overall the mean (SD) %pred FEV1 was 72.9% (22.0). The mean (SD) predicted FEV1 for the improved, stable, and worsened groups was 72.0% (18.6), 71.3% (20.0), and 70.6% (23.5) respectively. Overall, 40% of participants were classified as GOLD grade 1 (FEV1 ≥80% predicted),5 including 37%, 40%, and 38% of participants in the improved, stable, and worsened groups, respectively. In addition, 50 (60%) participants overall, including 53%, 60%, and 65% of participants in the improved, stable, and worsened groups, respectively, were considered to be at low risk of exacerbations (GOLD Group A).5
Of the 84 participants enrolled in the study, 68 (81%) had ≥25% adherence in terms of CAT score completion and were included in the ≥25% adherent subgroup. Baseline demographics and clinical characteristics for this subgroup, categorized by CAT score evolution group, are shown in Table 1.
COPD Assessment Test Score Evolution
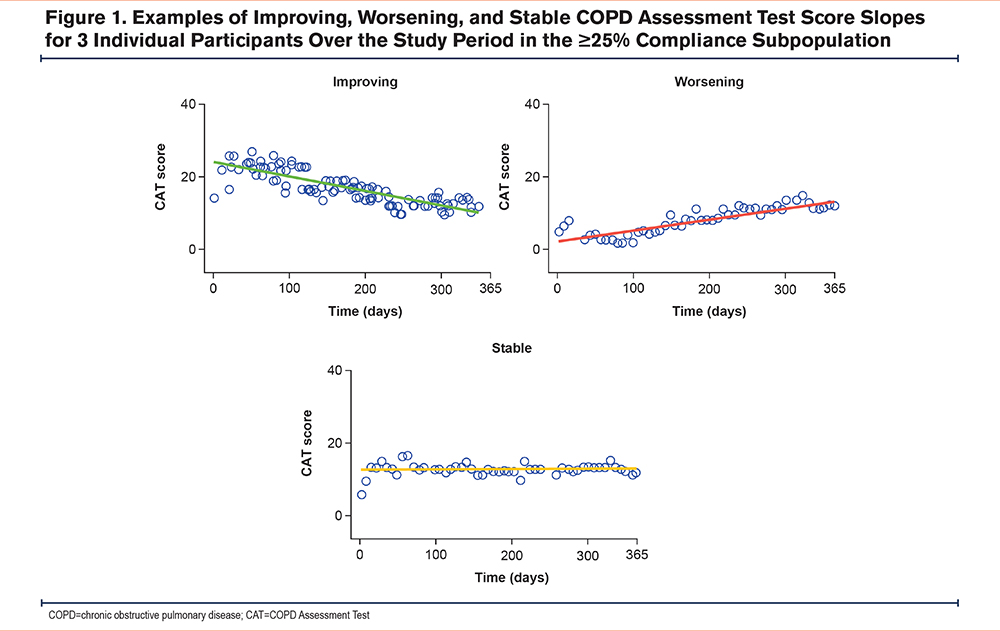
The evolution of CAT scores over time for both the overall population and the ≥25% compliance subgroup showed substantial variation between individual participants. Data from participants selected as representative examples of the 3 patterns of evolution are shown in Figure 1. Individual CAT score trajectories are shown in Supplementary Figure 1 in the online supplement. Linear regression at an individual patient level showed that 42% of participants had CAT scores indicating worsening health status, 35% had scores showing little change, and 23% had scores indicating improving health status.
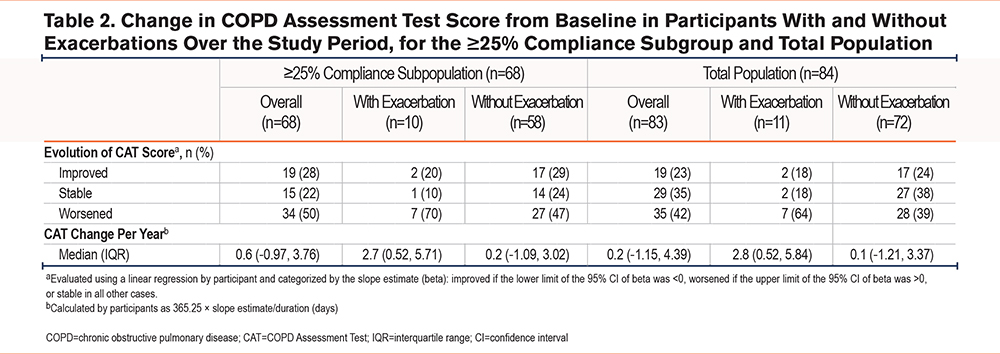
In the ≥25% adherence subgroup, 50%, 22%, and 28% of participants had CAT scores indicating worsened, stable, or improved health status, respectively (Table 2). The median (interquartile [IQR]) slope for CAT score change per year in the ≥25% adherence subpopulation over the study period was 0.6 (-1.0–3.8) units/year. Participants with CAT scores indicating improvement had a numerically shorter mean duration of COPD, and higher initial mean modified Medical Research Council and CAT scores than participants in the stable or worsened groups (Table 1).
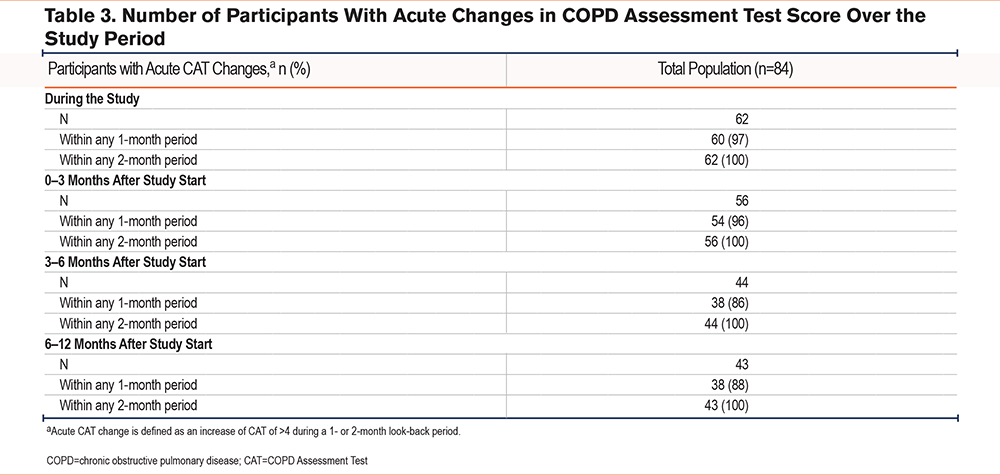
Over the course of the study, 62 (74%) participants experienced a change in CAT score of >4 units over the look-back periods of 4 and 8 weeks. Most of these changes (n=60 [81%]) were within a 4-week period (Table 3).
Association Between COPD Test Assessment Score Changes and Exacerbation Risk
Among participants in the ≥25% adherent subpopulation who had an exacerbation at some point during the study period (n=10), CAT score indicated a worsened health status for 7 (70%) participants compared with 27 of 58 (47%) participants who did not have an exacerbation (Table 2). The median (IQR) slope for CAT change per year over the study period was 2.7 (0.5–5.7) units/year for participants with exacerbations and 0.2 (-1.1–3.0) units/year for those without exacerbations.
The risk of an exacerbation increased per unit increase in CAT score over 1 year for both total exacerbations and moderate exacerbations (hazard ratio [HR]: 1.13 [95% CI: 1.03, 1.24]) and severe exacerbations (HR: 1.22 [95% CI: 1.05, 1.41]) (Table 4).
Learning Effect to Complete the COPD Assessment Test
A total of 6 (7%) participants showed evidence of a learning effect across the first few measurements at the start of the study, which can be seen for individual participants in Figure 1. The effect was observable in both directions and seemed to be unrelated to the CAT slopes after that initial period. The median learning time was 13.3 days (IQR 8.9–64.8).
Trajectory Analysis
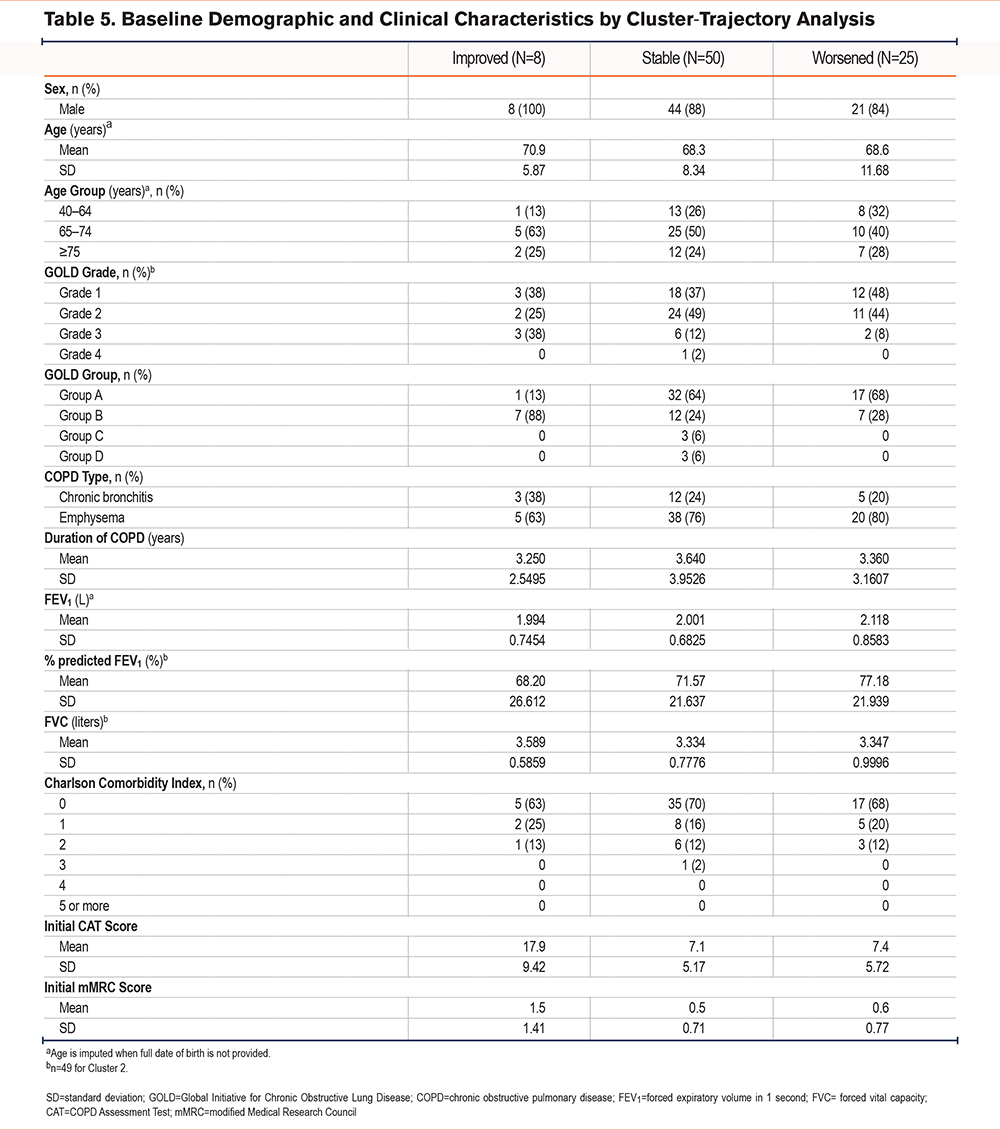
The trajectory analysis identified 3 clusters of participants (Figure 2) that fit the pattern of the CAT score evolution groups, with clusters representing improved, worsened, and unchanged (stable) health status. Participants in the cluster showing improvement had numerically fewer moderate/severe exacerbations in the previous 12 months and higher initial CAT scores. The %pred FEV1 and forced vital capacity were all higher in the improved cluster compared with those in the worsened cluster (Table 5).
Discussion
This study shows that the CAT may detect trends in health status using weekly scores collected over one year and help define a patient’s disease trajectory as worsening, stable, or improving. This may support a previous finding of a link between the progression of the CAT score and patient health status in a telemedicine setting.11 A significant positive association was observed between worsening CAT scores over time and the occurrence of both moderate and severe COPD exacerbations, with a 13% increased risk of moderate exacerbations, and a 22% increased risk of severe exacerbations observed for each unit increase in CAT score over one year. These findings may indicate that the use of CAT via a smartphone app allows regular telemonitoring of COPD-related health status, enabling patients and/or physicians to identify a pattern of worsening during routine COPD management. This could provide an opportunity for earlier treatment of symptoms, improving patient outcomes.21
The significant positive association between CAT score worsening and exacerbation occurrence may be because exacerbations worsen the CAT score for several days,22 and worsen health status for several weeks following the acute event.23 It is also known that higher CAT scores are a risk factor for exacerbations.10,24 Visual observation of the CAT slopes did not demonstrate a change in CAT before or after an exacerbation, however, we did not perform formal statistical comparisons between the CAT slope before and after an exacerbation, as the low number of events limited this analysis.
Using a change of >4 units over a look-back period of 4 weeks, we observed acute changes in 74% of participants, but this approach may miss exacerbations since a previous study showed a 4-unit recovery following an exacerbation after approximately 8 days and a mean change in CAT score of approximately 5 units between the stable state and an exacerbation.22 Furthermore, our findings suggest that changes of >4 units can occur in an individual over a period of up to a month even in the absence of an acute exacerbation. This is important because most studies measure CAT change between 2 time points, for example, beginning and end. However, this study shows that there are between-measurement changes that are clinically significant in magnitude but may be due to normal day-to-day variation coupled with measurement error. Trends based on weekly measurements should provide greater precision in estimates of change over time because they use a larger number of measurements, which may increase the CAT’s sensitivity to identify worsening or improving health status.
When using this type of methodology, adherence and the possibility of a learning effect should be considered. We used a cut-off of ≥25% adherence of regular data collection for this analysis to ensure that we had sufficient data to reliably estimate the slope of change over time, and 81% of participants were able to achieve this. The learning effect was not observed frequently, and the median time was approximately 2 weeks but, in the context of a clinical study, a decision to ignore the first 4 weeks (if using weekly measurements) could be prespecified. After any initial learning effect, the slope of change over time in this cohort study appeared to be largely linear. This contrasts with findings, often seen in pharmaceutical trials, where a rapid initial benefit may occur at the start of treatment, followed by stability or worsening.25,26 However, a nonlinear initial response should not be an obstacle to the use of CAT diaries, since appropriate methods can be used to take these initial effects into account.
The use of telemedicine to collect multiple CAT measurements may have significant application in future studies of COPD because of the precision permitted by multiple measurements and the ability to categorize patients into 3 defined groups using preset criteria. Identification of patients exhibiting deteriorating health status versus those who are stable or improving is clinically important due to the heterogeneity seen in COPD symptoms and response to treatment. The slope of the CAT score over time has already been shown to be affected in a disease management system.12 The methodology may also have application to predict medium-term outcomes. Future research could explore whether a 4–6 week recording period may predict deterioration assessed using a composite outcome measure such as clinically important deterioration.27
A strength of this study is that it extends observations from a study in Switzerland to participants in Japan. The participants recruited to this study had better lung function than those in the Swiss study11 (predicted FEV1 73% versus 38%), but a similar pattern of results was seen in both studies, suggesting that weekly CAT measurement may be effectively implemented in different health care settings and is not affected by COPD severity.
There are also limitations to this methodology; participants and physicians included in this study were selected from only 6 sites employing the YaDoc platform. However, these sites covered a diverse area and rural/urban locations in Japan. Some participants who are not accustomed to using mobile phone applications may have been less willing to participate, so the study population may be early adopters of the technology. Finally, while the trajectory analysis identified 3 clear patient clusters, the composition of these clusters may have been affected by a small learning effect in some participants, as well as by participants with particularly high or low baseline scores. The relatively small sample size in each individual cluster, as well as the overall sample size, should also be considered when making conclusions. However, the sample size was larger than that of the Swiss study,11 and large enough to identify health status patterns among the ≥25% adherent subgroup with some confidence. The low number of exacerbations is a weakness, but the relationship between CAT change and exacerbations was not the primary hypothesis under test and the exacerbation analysis should be considered as hypothesis generating.
Conclusions
The study shows that measurement of trends in CAT scores over time, obtained using weekly scores collected via telemedicine, may provide insights into medium-term COPD trajectories by identifying a pattern of worsening and alerting patients and/or physicians. This will be important for future studies where the identification of patients exhibiting deteriorating health status versus those whose status is stable or improving will provide insights into response to treatment. Prospective treatment trials are required to confirm these findings. Future studies could also examine CAT items separately for nonrespiratory versus respiratory items, as well as to examine what drives fluctuations in CAT scores.
Acknowledgements
Author contributions: TI, PJ, TM, YS, and TN contributed to the conception and design of the study. MS, OH, MM, MK, AM, and KT contributed to the acquisition of data. TS, TI, PJ, TM, YS, and TN contributed to the data analysis and interpretation.
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Christopher Heath, PhD, at Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK. We thank the participants and physicians/facility staff in Japan who participated in this study.
Data Sharing: Anonymized individual participant data and study documents can be requested for further research from https://www.gsk-studyregister.com/en/
Declaration of Interests
MS, OH, MM, MK, AM, and KT report having received grants from the GSK group of companies for the conduct of this study. YS reports having received personal fees from the GSK group of companies during the conduct of the study, and lecture fees from AstraZeneca, Novartis, and Boehringer Ingelheim. TN is an employee of GSK. TM is an employee of GSK and holds stocks/shares. TI and TS are former employees of GSK. PJ is an Emeritus Professor of Respiratory Medicine at St George’s, University of London, and a former full-time employee of GSK at the time of protocol development and contributed to study design and protocol on behalf of GSK. He is a part-time consultant at GSK and holds stocks/shares.