Running Head: Herpes Zoster Risk With Inhaled Corticosteroid Use
Funding Support: This work was funded by a research grant from Boehringer Ingelheim Pharmaceuticals, Inc.
Date of Acceptance: March 8, 2024 | Publication Online Date: March 12, 2024
Abbreviations: BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; HR=hazard ratio; HZ=herpes zoster; ICS=inhaled corticosteroid; RR=relative risk; SD=standard deviation
Citation: Yawn BP, Callen E, Gaona-Villarreal G, Shaikh A, Pace WD. Increased herpes zoster risk with inhaled corticosteroid use for those with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2024; 11(3): 303-306. doi: http://doi.org/10.15326/jcopdf.2023.0478
Introduction
Herpes zoster (HZ) or shingles is a common painful condition that occurs in about one-third of the general population by age 80 years. While for many shingles is limited to a few days to a 2-week long symptomatic prodromal period followed by 1 to 4 weeks of painful rash, many older individuals will experience 1 or more HZ complications including HZ opthalamicus and postherpetic neuralgia that can linger for months with a marked decline in the person’s quality of life and ability to continue usual activities.1,2 Several chronic medical conditions (rheumatic, inflammatory bowel, and chronic lung diseases including chronic obstructive pulmonary disease [COPD]) and associated treatments such as corticosteroids have been shown to increase the annual risk of HZ by 20% to 60%.3
Up to 75% of people diagnosed with COPD receive daily inhaled corticosteroid (ICS) therapy often augmented over several years by bursts of oral corticosteroid treatment for exacerbations. However, studies reporting on the risk of HZ in those with COPD exposed to daily ICSs4-8 only provide the relative risk (RR) or hazard ratio (HR) in comparison to individuals without COPD. This brief report compares groups with and without ICS exposure within the COPD population to better determine the risk of HZ when adding ICSs to COPD therapy. This is especially important since real-world data shows that many people with COPD are prescribed a daily ICS without meeting the recommendations for inclusion of an ICS in COPD maintenance therapy—frequent COPD exacerbations, high total eosinophil count, or concomitant asthma and COPD.4,9
Methods
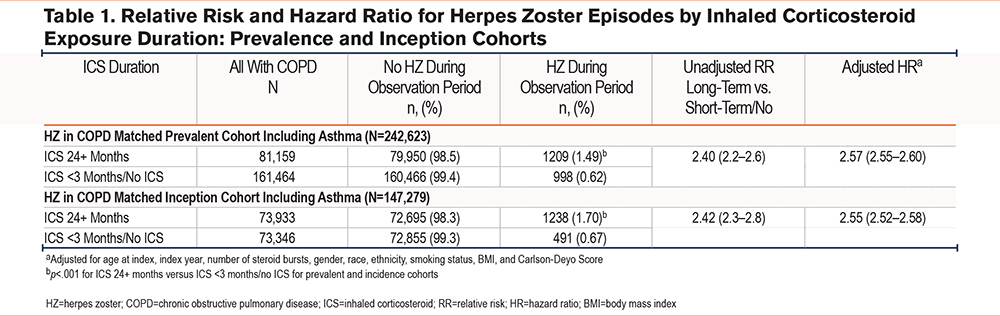
To test the hypothesis that a longer duration of an ICS as part of COPD therapy is associated with increased HZ risk using real-world data from the DARTNet Practice Performance Registry10 and selected additional databases, we compared the risk of HZ in people with COPD and no or <3 months (short-term/no) versus 24+ months (long-term) ICS exposure. The DARTNet Practice Performance Registry is a Centers for Medicare and Medicaid Services quality improvement registry. In the time frame included in this study, over 7000 clinical organizations from solo clinicians to large integrated health care systems provided electronic health record data for various periods of time.11,12 All individuals in the cohorts had a clinical diagnosis of COPD based on: (1) two separate visits with a COPD diagnosis separated by at least 3 months; (2) one visit for COPD with a prescription for a daily COPD maintenance medication (long-acting muscarinic antagonist, long-acting beta2-agonist, ICS, or any combination of those); or (3) a hospitalization for COPD. We developed both a COPD prevalence cohort, (had a COPD diagnosis at entry into the observation period) and an inception cohort, (had a new diagnosis of COPD 6+ months after entry into the database or 2 visits within the first 6 months of observations without a COPD diagnostic code). The individuals within each cohort were propensity matched, through the use of genetic exact matching, based on multiple demographic factors. The duration of ICS exposure was based on prescribing data, and oral corticosteroid exposure was collected for all groups. HZ vaccination and spirometry data were not available. For analysis, the people who previously had HZ were dropped after matching.
Data are presented separately for the 2 cohorts comparing HZ occurrence for the long-term versus short-term/no ICS-exposed individuals. The first occurrence of an HZ episode during the observation period was based on the presence of any HZ-related code with no HZ codes in the prior 12 months. Recurrences were not assessed. Simple RR (univariate analysis, not corrected for demographics or bursts) and HR (Cox proportional hazards) from modeling, to account for differing demographics and numbers of oral corticosteroid bursts per person, were calculated. Burst frequency is important for total corticosteroid exposure and as a proxy for COPD exacerbations and COPD severity. Mean and median duration of follow-up were 3.98 and 4.00 years respectively with a standard deviation (SD) of 3.06.
Results
For the prevalence cohort, the long-term ICS exposure group versus short-term/no exposure (N=242,623) included more women (57.4% versus 53.7%, P<.001) with a younger mean age (66.8 years [SD 10.4] versus 68.2 years [SD 11.3], p<.001) and fewer with Charlson-Deyo scores of >2 (22.8% versus 25.8%, P<.001). The demographics of the inception cohort (COPD diagnosed after 6 months in the database, N=147,279) were similar with more women (58.0% versus 54.3%, p<.001), lower mean age (66.8 years [SD 10.5] versus 68.6 years [SD 11.2], p<.001), and fewer with a baseline Charlson-Deyo score of >2 (24.1% versus 31.0%, p<.001) in long-term versus short-term/no ICS exposure groups.
The risk of HZ was significantly greater with longer exposure to an ICS in both the prevalence and inception cohorts: RR = 2.40 (95% confidence interval [CI] 2.20–2.60) for the prevalence cohort and RR = 2.42 (95% CI 2.30–2.80) for the inception cohort. The HR after adjusting for demographic factors were similar: HR=2.57 (95% CI 2.55–2.60) for the prevalence cohort and 2.55 (95% CI 2.52–2.58) for the inception cohort (Table 1). The absolute risk was a 1.03% increase in risk of HZ for the inception cohorts over an average period of 4 years with a number needed to harm of 100.
Discussion
This is one of the first studies to report on the increased risk of HZ in those with COPD with long-term versus short-term/no ICS exposure. Comparisons with previously published risk assessments are limited since most studies reported comparative risks of ICS exposure in cohorts with and without COPD. Yang et al5 reports HRs of 1.67 and 2.09 for those with COPD without and with ICS exposure compared to a matched Taiwanese population without COPD. From Spain, Munos-Quiles et al7 reports RRs of 1.45 and 1.61 again comparing those with COPD without and with ICS exposure to a matched group without COPD. In a German claims-based study, Batram et al8 reported an increased risk of HZ in people with COPD prescribed 1 or more bursts of oral steroids. None of these studies are limited to COPD populations and do not report the risk from ICSs added to the risk of having COPD. Our study was limited by all the limitations of using EHR data, lack of HZ vaccination data, and no spirometric confirmation of COPD. However, these were likely to be similar for all people with clinical diagnoses of COPD regardless of ICS exposure. We are also unable to report whether the HZ risk continues to increase with more years of ICS exposure.
Within these COPD populations, an increased risk of HZ was associated with a longer duration of ICS exposure emphasizing the importance of balancing the risk and benefits from adding an ICS to COPD maintenance therapy.9 Based on local vaccination guidelines, clinicians should consider recommending or administering HZ vaccine to their patients with COPD, especially those on a long-term ICS.3,13
Acknowledgements
Author contributions: All authors were responsible for conception and design of the study. ECallen, GGaona-Villarreal, and WPace were responsible for data acquisition; BYawn, ECallen, GGaona-Villarreal, and WPace were responsible for data analysis and interpretation; and all authors were responsible for writing or substantially revising the manuscript prior to its submission.
Declaration of Interests
BP Yawn reports receiving honoraria from AstraZeneca, GlaxoSmithKline, Merck, TEVA, and Boehringer Ingelheim for consulting and work on advisory boards and from AstraZeneca and GlaxoSmithKline for travel to presentations. BP Yawn is on the Medical and Scientific Advisory Committee of the COPD Foundation (unpaid). W Pace has received research grant support from Boehringer Ingelheim (outside of this project), AstraZeneca, the Patient-Centered Outcomes Research Institute, the National Institutes of Health, ONC, the Centers for Disease Control and Prevention, and Tabula Rasa Healthcare. W Pace is on the Advisory Board and is an Executive Committee member (unpaid) for the COPD Foundation360 Network and owns stock through a trust in Johnson and Johnson, Eli Lily, Novo-Nordisk, Pfizer, Novartis, Moderna, and Amgen. A Shaikh was affiliated with Boehringer Ingelheim during the study and is now an employee of Sun Pharma. E Callen and G Gaona-Villarreal have nothing to declare.