Running Head: Iron Deficiency and COPD
Funding Support: The study “Long-term outcome of oxygen supplementation on cardiovascular comorbidity, anemia and psychological distress in chronic obstructive pulmonary disease” was sponsored by the University of Oslo.
Date of Acceptance: March 26, 2024 | Publication Online Date: April 3, 2024
Abbreviations: BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disorder; CRP=C-reactive protein; CVD=cardiovascular disease; FEV1=forced expiratory volume in 1 second; FEV1%pred=percentage of predicted forced expiratory volume in 1 second; FVC=forced vital capacity; HR=hazard ratio; LTOT=long term oxygen treatment; M=number of deaths; mMRC=modified Medical Research Council; MR=mortality rates per 1000 years; MRR=mortality rate ratios; N=number of participants; OR=odds ratio; SD=standard deviation; TSat=transferrin saturation; WHO=World Health Organization
Citation: Hardang IM, Søyseth V, Kononova N, Hagve T-A, Einvik G. COPD: iron deficiency and clinical characteristics in patients with and without chronic respiratory failure. Chronic Obstr Pulm Dis. 2024; 11(3): 261-269. doi: http://doi.org/10.15326/jcopdf.2023.0477
Online Supplemental Material: Read Online Supplemental Material (264KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic disorder with altered architecture and function of the airways and alveoli.1 The severity of COPD is assessed both by pulmonary function tests and by clinical features, such as symptoms and exacerbation frequency. End-stage COPD may be accompanied by chronic respiratory failure, which has a high mortality rate. Inflammation and hypoxemia are pathophysiological factors in COPD having increasing importance with increased disease severity and may contribute to the range of comorbidities frequently associated with COPD.
Amongst comorbidities, anemia and iron deficiency have in some studies been shown to have a higher prevalence in COPD than in the normal population.2 There is a biological rationale for an association between COPD and iron metabolism disturbance; chronic inflammation may cause iron deficiency and/or anemia by restraining iron availability through retention of iron in the storage and reduced dietary iron uptake,3 while hypoxemia may cause secondary polycythemia,4 which is iron consuming (Figure 1). The clinical relevance of iron deficiency relates to its role as a pre-anemic condition,5 and it may aggravate the symptoms associated with COPD, particularly in the presence of hypoxemia.6 Thus, it could be advocated that iron deficiency in COPD may be included in the concept of treatable traits, as iron supplementation is an important and applicable treatment opportunity in COPD patients with reduced iron storage.
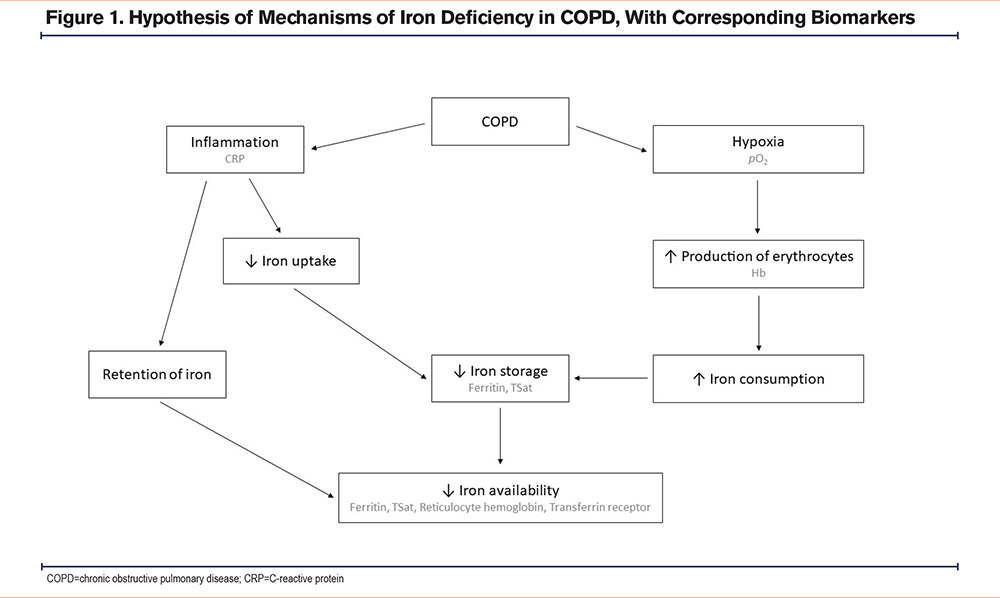
However, current evidence for the role of iron deficiency in COPD has some limitations. The prevalence of iron deficiency varies greatly between the studies, and they are often not comparable as they differ in both study population and diagnostic criteria for iron deficiency. Chronic hypoxemia is of particular interest as it may attenuate some of the effects of inflammation on iron metabolism by reducing the production of hepcidin, an iron regulatory peptide.7,8 Furthermore, the putative role of iron deficiency regarding mortality in COPD is yet uncertain.
Subsequently, the aims of the study were: (1) to assess the prevalence of iron deficiency by 3 different diagnostic criteria in participants with COPD with and without chronic respiratory failure compared with a control group without COPD, (2) to relate iron deficiency defined by different diagnostic criteria to clinical and biochemical characteristics, and (3) to assess the prognostic influence by iron deficiency on mortality in patients with COPD.
Materials and Methods
Design and Participants
The “Long-term outcome of oxygen supplementation on cardiovascular comorbidity, anemia, and psychological distress in chronic obstructive pulmonary disease” study was an observational cohort study conducted at Akershus University Hospital. All participants were included between September 2013 and March 2017. The study ended in April 2020.
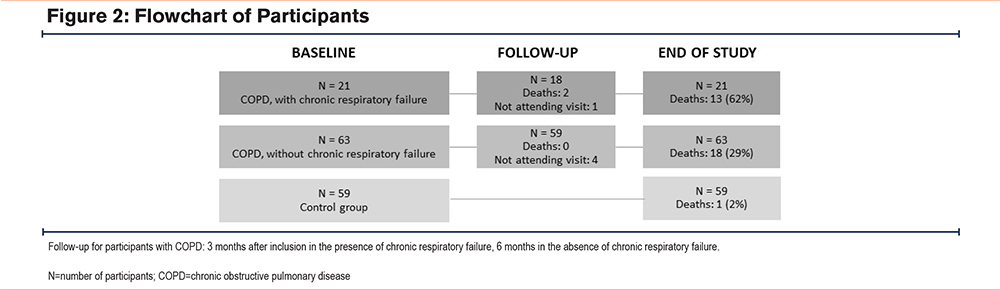
The inclusion criteria were separated into 2 strata: stable COPD with and without chronic respiratory failure. Respiratory failure was defined as arterial oxygen tension < 8.0 kPa or carbon dioxide tension > 6.7 kPa. The participants with chronic respiratory failure were referred to our department for initiation of long-term oxygen treatment (LTOT), and thus, none used LTOT at baseline. The diagnosis of COPD was based on recommendations by the Global initiative of chronic Obstructive Lung Disease (GOLD),1 as assessed by the responsible clinical physician. Patients attending the outpatient pulmonary clinic and the outpatient pulmonary rehabilitation program were assessed for eligibility whenever the researcher (N. Kononova) was available. Furthermore, a control group, randomly drawn from the National Population Registry, without COPD (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio above the lower limit of normal) and age ≥ 40 years was recruited from the hospital’s catchment area. Exclusion criteria were cognitive deficits resulting in inability to give informed consent, acute exacerbation of COPD in the last 4 weeks, or presence of advanced cancer. The participant flow is shown in Figure 2.
All participants provided written informed consent prior to initiation of data collection, and the study was approved by the regional medical ethical committee (2013/1006) and the local data protection officer.
Data Collection
The following examinations were performed for all participants: Pre and postbronchodilatory spirometry, height and weight, venous blood samples, questionnaire of current and previous smoking habits, dyspnea, and medical history. Arterial blood gas was examined in participants with COPD only. Venous blood samples were repeated at a follow-up visit, which, according to the clinical guidelines in the department, took place after 3 months for participants with chronic respiratory failure and after 6 months for participants without chronic respiratory failure. There was no follow-up visit for the controls. From medical history, we reported previous cardiovascular disease as a composite of angina, myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, aortic aneurysm, carotid artery stenosis, or peripheral artery disease. Patients were asked whether they became breathless when walking indoors on the same floor, and whether they were breathless when at rest, similar to the questions in the modified Medical Research Council (mMRC) dyspnea scale grades 3 and 4. Body mass index (BMI) was calculated as weight (kg) / height2 (m). Spirometry was performed according to recommendations by the European Respiratory Society and American Thoracic Society Guidelines.9 We registered FEV1, FVC, and the FEV1/FVC ratio. Partial pressure of arterial oxygen and carbon dioxide were obtained from arterial blood samples analyzed on an ABL 800 blood gas analyzer. Venous blood samples were analyzed immediately at the local clinical laboratory. C-reactive protein (CRP), ferritin, transferrin, and iron were analyzed on Vitros 5600. Hemoglobin and reticulocyte hemoglobin were analyzed on a Sysmex XN-10 or Sysmex XN-20 hematology analyzer. Transferrin receptor was analyzed on cobas c 501. The group level of CRP included measurements with results < measuring range, assigned a value of 0.1mg/L.
All participants were followed until death or the end of the study in April 2020, with median (25th–75thpercentile) follow-up period of 4.4 (4.1–4.9) years. The date of any death in the follow-up period was collected from the National Death Registry of Norway.
Definition of Iron Deficiency
Based on international expert opinion,10 common practice in studies involving participants with COPD, and the findings of Grote Beverborg et al,11,12 we chose the following 3 diagnostic criteria for iron deficiency: (1) plasma ferritin < cutoff (100µg/L in participants with inflammation, 30µg/L in participants without inflammation)5; (2) plasma transferrin saturation (TSat) < 20%10; or (3) a combination of plasma ferritin < cutoff and plasma TSat < 20%.12 For the main analyses, the presence of COPD implied inflammation, while CRP > reference range implied inflammation in the control group.
Statistical Analyses
Comparison of levels of iron-related parameters between groups was performed using Mann-Whitney-U test (non-normally distributed continuous variables), while a comparison of iron deficiency prevalence between groups was performed using χ2-test and Fischer’s exact test. We also compared the prevalence of iron deficiency in which the presence of inflammation, and therefore, ferritin cutoff, was defined only by CRP > reference range. Uni- and multivariable logistic regression analyses were used to calculate odds ratios for iron deficiency by the following covariates: sex, age, hemoglobin, CRP, BMI, FEV1, arterial oxygen tension, current smoking, and cardiovascular disease. Covariates that in univariable analyses were associated with iron deficiency (p<0.05), were selected for the final multivariable analyses. The included covariates did not display multicollinearity and were chosen under the assumption that they may be associated with iron metabolism and/or severity of COPD. Crude mortality rates and mortality rate ratios were calculated for iron deficiency and covariates assumed to influence mortality. For iron deficiency criteria, the strength of the associations between baseline and follow-up data with mortality in participants with COPD were analyzed by mixed-effects univariate Cox regression analyses, and adjusted for covariates if associated with a p-value <0.05. All deaths are registered in the National Death Registry of Norway, and participants not attending follow-up visits were included in the Cox regression analyses with their baseline iron status. Missing data are indicated in the tables by a superscript letter pointing to a note. Stata 17.0 was used for all data analyses.
Results
The baseline characteristics for the total sample (N=143) and stratified according to subgroup (COPD with chronic respiratory failure [N=21], COPD without respiratory failure [N=63], and control group [N=59]) are presented in Table 1.
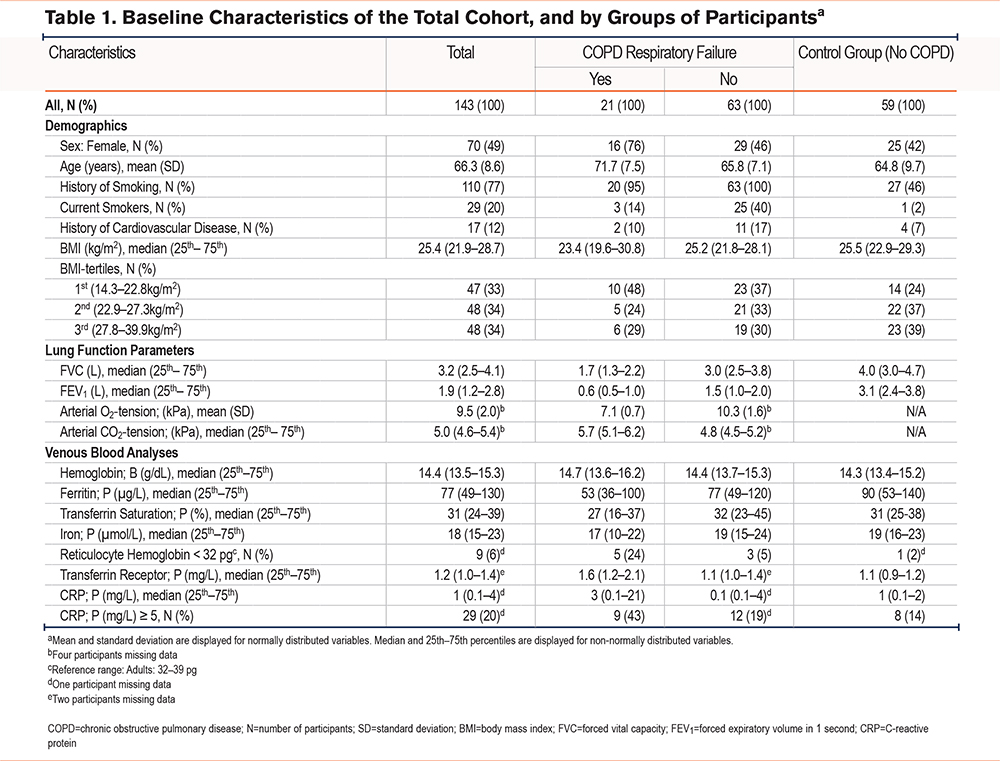
The control group and the nonrespiratory failure group were similar regarding demographic data, except for smoking. The chronic respiratory failure group differed from the other 2 groups by having an older population of predominantly females. The levels of biochemical and hematological parameters are displayed in Table 1. The ferritin level was significantly lower (p<0.05) in the group of chronic respiratory failure compared with controls, while the level of transferrin receptor was significantly higher (p<0.01) in the group of chronic respiratory failure compared with the other 2 groups. Only 2 out of 143 study participants, both with chronic respiratory failure, had transferrin receptor level > reference range. The levels of hemoglobin and TSat were not significantly different across groups. The proportion of participants with reticulocyte hemoglobin < reference range was significantly higher (p<0.05) in the group of chronic respiratory failure compared with the others.
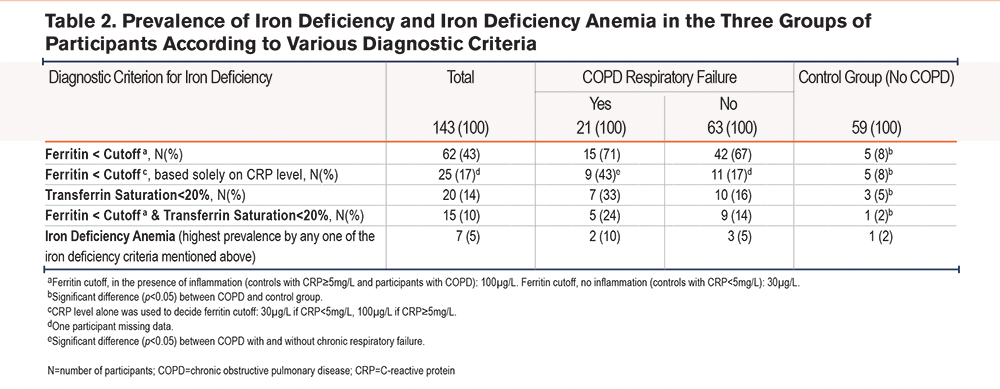
The prevalence of iron deficiency varied according to the diagnostic criteria and between groups of participants (Table 2). Ferritin < cutoff, whether the cutoff was determined by the presence of COPD or solely by CRP level, was the criterion resulting in the highest prevalence of iron deficiency. The combination of ferritin < cutoff and TSat < 20% yielded the lowest prevalence. One-fourth of the participants with TSat < 20% had high or normal ferritin levels, and around one-fourth of the participants with ferritin<cutoff, also had TSat < 20% (Supplemental Table 1 in the online supplement). In our study population, consistent for all diagnostic criteria, was the finding of increased prevalence of iron deficiency with more severe COPD.
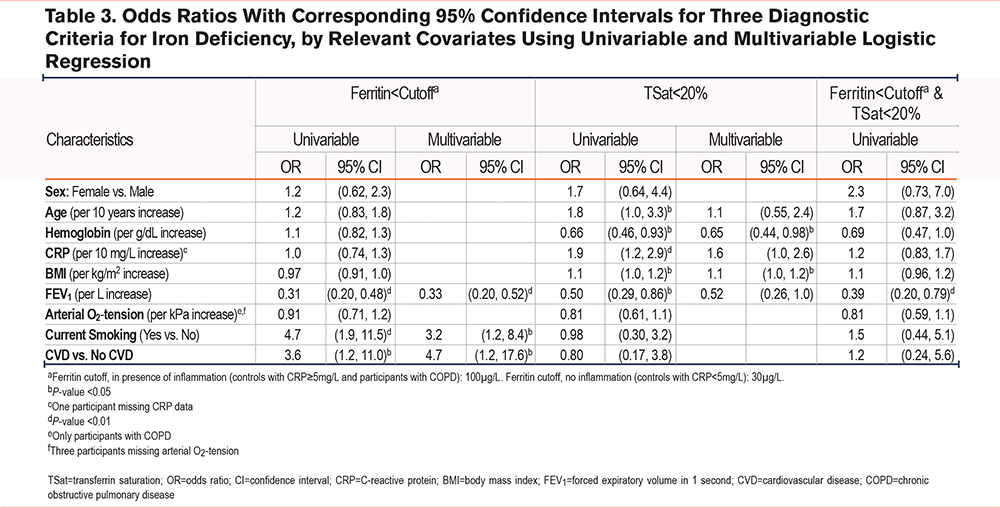
When assessing the relationship between iron deficiency and clinical variables (Table 3 and Supplemental Table 2 in the online supplement) we found that ferritin < cutoff was associated with decreased FEV1 and was more frequent in current smokers and patients who had a history of cardiovascular disease compared with their counterparts. TSat < 20% was associated with a decreased hemoglobin level and increasing BMI. However, among the participants with an elevated BMI, TSat < 20% was significantly more prevalent in the presence of chronic respiratory failure than otherwise. In the group of respiratory failure, 5 out of 6 (83%) with BMIs in the upper tertile also had TSat < 20%. The corresponding numbers were 4 (21%) in the group of COPD without respiratory failure, and 1 (4%) in the control group. The combined criterion of ferritin < cutoff and TSat < 20%, was univariably associated only with FEV1, and multivariable analysis was, therefore, not performed. None of the diagnostic criteria of iron deficiency were associated with age or sex in multivariable analyses.
The prevalence of abnormal hemoglobin levels varied within the groups of participants. Anemia, as defined by the World Health Organization,13 was uncommon in the group of participants as a whole. Only 11 (8%), 3 to 4 participants in each of the groups, out of a 143 total participants had anemia (Supplemental Table 3 in the online supplement). Elevated levels of hemoglobin were found in 9 (6%) participants. These were all females. They had COPD, and the majority had chronic respiratory failure and hypoxemia. Compared with the participants with anemia, there was a greater proportion with ferritin < 100µg/L among the participants with elevated hemoglobin. However, none of the participants with elevated hemoglobin had TSat < 20%.
Iron Deficiency and Mortality
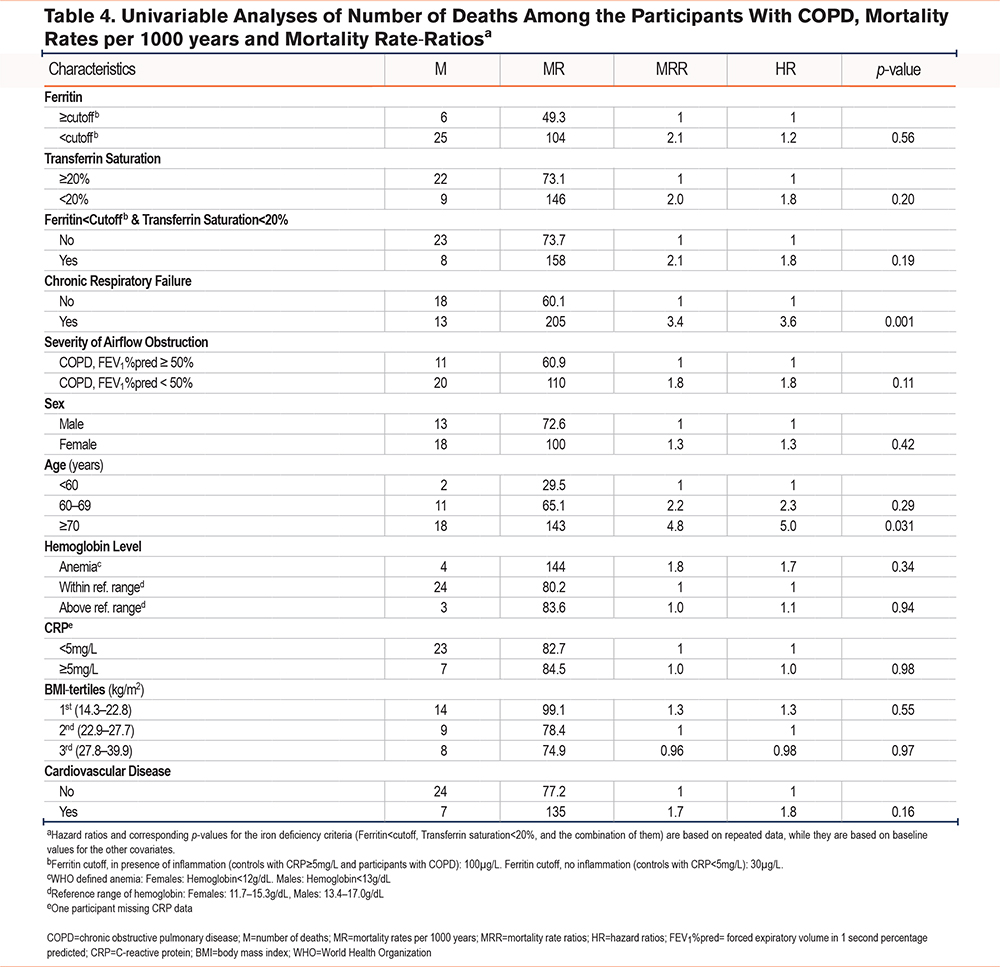
None of the 3 criteria of iron deficiency was associated with increased mortality in the participants with COPD in univariable analyses, thus, adjusted analyses were not performed. Among covariates, higher age and chronic respiratory failure were, as expected, associated with increased mortality. The crude mortality rates and rate ratios are shown in Table 4.
Discussion
The main findings in the present study are that iron deficiency was more common in individuals with COPD compared with controls and that the prevalence increased with severity of COPD in our study population, consistently across 3 definitions of iron deficiency. Ferritin < cutoff gave the highest prevalence estimate of iron deficiency and was associated with known pathophysiological factors in COPD. In contrast, TSat < 20%, or the combination of ferritin < cutoff and TSat < 20%, were to a lesser degree associated with COPD severity. None of the iron deficiency definitions were associated with mortality in the participants with COPD.
Which biomarker and which cutoff to use to diagnose iron deficiency in patients with COPD are subject to controversy. The prevalence of iron deficiency as determined by the gold standard, i.e., iron staining of the bone marrow, has not been assessed in large samples of this particular population. Furthermore, co-occurring conditions, such as chronic inflammation and hypoxemia, are prevalent in COPD and may disturb the interpretation of iron deficiency biomarkers. Therefore, the diagnostic criteria for iron deficiency in otherwise healthy persons are inadequate for patients with COPD. Independently of the different criteria used in this study, we report that iron deficiency is more common in individuals with COPD than in non-COPD individuals, in accordance with previous studies.2 We found increased prevalence of iron deficiency across the severity of COPD defined by airflow limitations. However, grading of COPD severity by airflow limitation or by hypoxemia may constitute different pathophysiological aspects of COPD with differing influences on iron metabolism. Thus, we extended our analyses to explore how the different criteria for iron deficiency were associated with clinical and pathophysiological aspects of COPD. We found that low ferritin was associated with current smoking, airflow limitation, and cardiovascular comorbidity, but not with hypoxemia. TSat < 20% was less prevalent than low ferritin, and not associated with any COPD severity parameters nor by CRP level > reference range.
Ferritin, being both an iron storage protein and an acute phase protein, increases both with inflammation and increasing iron storage. Assuming a similar iron status, we would thus expect ferritin to increase by severity of inflammation. However, the fact that ferritin tends to be lower in patients with chronic respiratory failure, i.e., with supposedly the highest degree of inflammation, may indicate iron deficiency in these patients.
Another novel analysis in the current study was to explore the combination of the 2 criteria ferritin < cutoff and TSat < 20% as an indicator of low iron storage. This concept was shown to be clinically meaningful and closely associated with bone marrow characterization of iron status in patients with heart failure.12 In our study, however, few participants fulfilled this combined criterion. The prevalence pattern of iron deficiency by this criterion followed the other criteria, i.e., higher prevalence in participants with COPD compared with the control group, and highest prevalence in the presence of chronic respiratory failure. However, the prevalence estimates were not significantly different between COPD patients with and without chronic respiratory failure, and we found no association with pathophysiological mechanisms except airflow obstruction.
Hemoglobin levels both below and above the reference range was common in patients with chronic respiratory failure as shown in Supplemental Table 3 in the online supplement. Chronic hypoxemia is a strong initiator of erythropoiesis, and we should expect polycythemia in patients with chronic respiratory failure. A hemoglobin level within the reference range may indeed indicate a clinically significant iron deficiency in patients with chronic respiratory failure. A discrepancy between the expected elevated and observed hemoglobin levels in patients with chronic respiratory failure indicates the clinical relevance of assessing iron status in this group. The majority of study participants with elevated hemoglobin, had ferritin < 100µg/L, indicating iron being used in erythropoiesis. In our study, low reticulocyte hemoglobin was common in patients with chronic respiratory failure, another indicator of reduced availability of iron in the bone marrow.
The increase of the level of transferrin receptors is caused by 2 phenomena that are demonstrated in participants with chronic respiratory failure: unmet need for iron in the bone marrow and increased production of erythrocytes. Therefore, it is surprising that only a few of the participants with chronic respiratory failure and suspected iron deficiency display transferrin receptor levels above reference range. However, the reference range may include values from persons with pre-anemic iron deficiency, as bone marrow examination has not been used to exclude it in the reference population. Thus, the transferrin receptor level may not be a sensitive indicator of pre-anemic iron deficiency and therefore, not a parameter to rely on to identify iron deficiency in COPD patients.
Finally, we found that iron deficiency was not associated with increased mortality in participants with COPD. This is in contrast to Schneckenpointner et al who reported TSat as an independent predictor of 48-months survival in a population of 185 patients with respiratory failure.14 Beverborg et al found that the combination of Tsat < 20% and ferritin ≤ 128µg/L was independently associated with mortality in a population of heart failure.12 The discrepancy in our study suggests that underlying COPD severity and comorbidities have a greater influence on mortality than iron deficiency does.
There are several limitations in the current study. First, the sample size was fitted for the main outcome in the LTOT study (change in cardiac biomarkers), and not for mortality. It was, however, comparable to several other studies in the field of iron deficiency and COPD, and sufficiently powered to assess continuous outcomes. Second, we did not include bone marrow analyses as a gold standard for iron depletion. Such examinations in a larger group of patients may clarify several of the uncertainties in this field. Third, unstable COPD, i.e., a phenotype with frequent exacerbation, was an exclusion criterion, thus, patients with a rapidly progressive disease with frequent hospital admissions were not included. Finally, prospective characterization of iron status, hematological variables, inflammatory markers and COPD severity would also increase the knowledge of the potential impact by iron depletion on COPD outcomes.
Conclusion
The prevalence of iron deficiency increased by increasing COPD severity irrespective of diagnostic criteria in our study population. When determined by ferritin<cutoff, iron deficiency was associated with bronchial obstruction, current smoking, and cardiovascular disease, while it was associated with reduced levels of hemoglobin and increased BMIs when determined by TSat < 20%. Iron deficiency was not associated with increased mortality in participants with COPD.
Acknowledgements
Author contributions: IMH, VS, TAH, and GE contributed to the conceptualization. IMH, VS, and GE contributed to the formal analysis. IMH, VS, TAH, and GE contributed to the methodology. IMH and GE contributed to the writing of the original draft. IMH, VS, TAH, NK, and GE reviewed and edited the manuscript. VS was in charge of the funding acquisition. VS and GE served as project administration. VS, TAH, and GE supervised. NK was in charge of data curation and investigation. GE contributed to the validation. All authors reviewed and approved the final manuscript submitted for publication.
Data Sharing: An anonymized data set may be made available upon request to the corresponding author. The current approval does not permit further novel analyses to be performed and published.
We thank the Clinical Trial Unit in the Department of Research, Akershus University Hospital for contributing to data collection, and the Department of Multidisciplinary Laboratory Medicine and Medical Biochemistry, Akershus University Hospital for blood analyses.
Declaration of Interests
GE has received unrestricted grants from Boehringer Ingelheim, Astra Zeneca, and ResMed for pulmonary research projects and has received honoraria from Astra Zeneca for expert panel discussion. All other authors have nothing to disclose.