Running Head: Blood Eosinophil Stability in Patients With COPD
Funding Support: This study was supported by the Faculty of Medicine, Prince of Songkla University, Thailand (grant number REC.64-408-14-1).
Date of Acceptance: April 1, 2024 | Publication Online Date: April 3, 2024
Abbreviations: BEC=blood eosinophil count; COPD=chronic obstructive pulmonary disease; CI=confidence interval; FEV1=forced expiratory volume in 1 second; FEV1%pred=forced expiratory volume in 1 second percentage predicted; FVC=forced vital capacity; GINA=Global Initiative for Asthma; GOLD=Global Initiative for Chronic Obstructive Lung Disease; ICC=intra class correlation coefficients; ICS=inhaled corticosteroid; IRR=incidence rate ratio; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; mMRC=modified Medical Research Council; SABA=short-acting beta2-agonist, SAMA=short-acting muscarinic antagonist; SD=standard deviation
Citation: Kaenmuang P, Navasakulpong A, Geater SL, Densrisereekul S, Juthong S. Blood eosinophil count stability and clinical outcomes in patients with chronic obstructive pulmonary disease in a high endemic area of parasitic infection: a prospective study. Chronic Obstr Pulm Dis. 2024; 11(4): 350-358. doi: http://doi.org/10.15326/jcopdf.2023.0492
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death, globally.1 The number of patients with COPD worldwide in 2019 was >384 million.2 In 2022, the number of patients diagnosed with COPD in Thailand exceeded 180,000 people.3 Eosinophilic airway inflammation can occur during exacerbation and stable disease. The blood eosinophil count (BEC) is an effective biomarker for predicting responsiveness to inhaled corticosteroids (ICS) in patients with COPD.4-9 BEC is currently widely used to determine appropriate ICS-based treatment in patients with eosinophilic COPD, especially patients with a BEC≥300 cells/μL. Although Thailand is an endemic area of parasitic infection, COPD patients with a BEC≥300 cells/μL also have more frequent exacerbations and a higher number of hospital admissions than non-eosinophilic patients. The BEC is a significant predictive factor for COPD exacerbation with an adjusted odds ratio of 3.91. Additionally, the mean baseline BEC in Thai patients with COPD is higher than that in other populations.10
Despite previous studies showing the reproducibility of blood eosinophil results in patients with COPD at both high and low blood eosinophil levels,11-14 there is ambiguity in the stability of blood eosinophils which could be affected by other factors such as exacerbation, corticosteroid use, and comorbidities.
To our knowledge, patients who have parasitic infections also exhibit elevated BECs. Endemic regions for parasitic infections notably include Africa, South America, and the Asia-Pacific area.15 Recent studies highlight a declining trend in the prevalence of parasitic infections among the Thai population.16,17 These reports affirm the enhancements in patient hygiene and health care education, marking a significant reduction as compared to earlier reports.18,19 However, Thailand is still identified as an endemic region for parasitic infections. Two prospective studies conducted in Thailand by Juthong et al and Saiphoklang et al have corroborated the presence of significantly high blood eosinophil counts in patients diagnosed with COPD, independently of any parasitic infection interference.10,20 However, there are no reports on the stability of BECs in Thai patients with COPD, who have unusual clinical characteristics and live in an endemic area of parasitic infection. Clinicians are reluctant to use blood eosinophil levels, as they question their stability and how they should be used in clinical practice.
We aim to explore the research question of whether the stability of BECs serves as a significant biomarker for forecasting clinical outcomes within regions highly endemic for parasitic infections.
This study prospectively explored the stability of BECs in Thai patients with COPD at the 6- and 12-month follow-ups and identified the association between blood eosinophil stability, exacerbation rates, and other clinical outcomes.
Materials and Methods
Study Population and Design
A prospective observational clinical study was conducted at a tertiary hospital, Songklanagarind Hospital (Songkhla, Thailand), from January 2022-January 2023. Enrollment took place from January 1-31, 2022. Each patient was followed for 12 months.
Patients were eligible for this study if they met the following criteria: (1) they were older than 40 years of age, and (2) they had been diagnosed with COPD according to the criteria described in the 2021 Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines.21
Eligible patients’ comorbidities were reviewed from an electronic-based health information system at Songklanagarind Hospital by 2 investigators (PK and SD). Patients were excluded if they had other conditions that could cause eosinophilia (e.g., parasitic infection, allergy, asthma, asthma-COPD overlap, autoimmune disease, or haematological malignancy).
To assess allergies, we interviewed the patients and reviewed the medical records of patients’ allergic histories to common allergens, such as house dust mites and foods, along with a history of allergic rhinitis and certain cases where serum-specific immunoglobulin E was presented.
Our evaluation of asthma and asthma-COPD overlap was followed by the Global Initiative for Asthma (GINA) and GOLD 2021 guidelines,21,22 focusing on clinical history, variable respiratory symptoms, variable expiratory airflow limitation, and confirmed by spirometry, bronchodilator reversibility testing, fractional exhaled nitric oxide, etc.
We investigated parasite infections through patients’ clinical histories and performed stool examinations to identify and exclude those with parasitic infections before enrollment. Patients who had previous acute exacerbations or had received systemic corticosteroid therapy within 4 weeks were also excluded.
BEC was measured at enrollment, and at 6 and 12 months. The recruited participants were classified into 3 groups according to their baseline BEC: BEC<100, 100-299, and ≥300 cells/µL. The stability of BEC was referred to the consistency of BEC in the same group over time. Persistent groups were defined based on the stability of BEC in the group after the 6- and 12-month follow-up periods.
Baseline demographic data including age, sex, smoking history, GOLD classification, modified Medical Research Council (mMRC) dyspnea scale, medication, and pulmonary function parameters were collected. The blood eosinophil stability was monitored throughout the study period. The association between blood eosinophil stability and exacerbation rates, including patient-reported outcomes, was recorded at the 6- and 12-month follow-ups.
This study was approved by the Human Research Ethics Committee at the Faculty of Medicine, Prince of Songkla University, Thailand (REC.64-408-14-1). All patients provided written informed consent before enrolling.
Statistical Analyses
Demographic data were reported as mean ± standard deviation or median with interquartile range according to the data type. Discrete parameters were presented as numbers and percentages. Differences in data were analyzed using Chi-squared and Fisher’s exact tests. The stability of BECs within each group were reported as the percentage of patients who maintained consistent BEC levels within the same group at follow-up intervals of 6 and 12 months.
Incidence rate ratio (IRR) was assessed through multilevel Poisson regression analysis to evaluate the differences of exacerbation rates across each persistent BEC group. Multilevel Poisson regression analysis was also used to compare the varied associations between blood eosinophil stability and exacerbation rates in variable and persistent BEC groups. We illustrated these associations by the mean predicted annual exacerbation rate. Statistical significance was set at p<0.05. Statistical analyses were performed using Stata/MP 16.0 for Mac (StataCorp LLC, College Station, Texas).
Results
A total of 280 patients with COPD who visited the outpatient department were screened. Of these, 256 patients were enrolled in this study. Four patients died during the follow-up period. Only 252 patients with COPD with complete follow-up were included in the analysis (Figure 1).
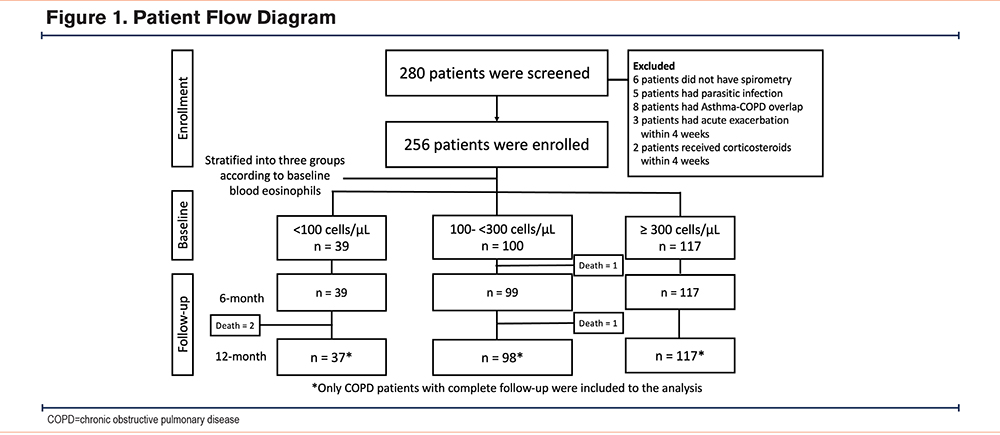
Patient Characteristics
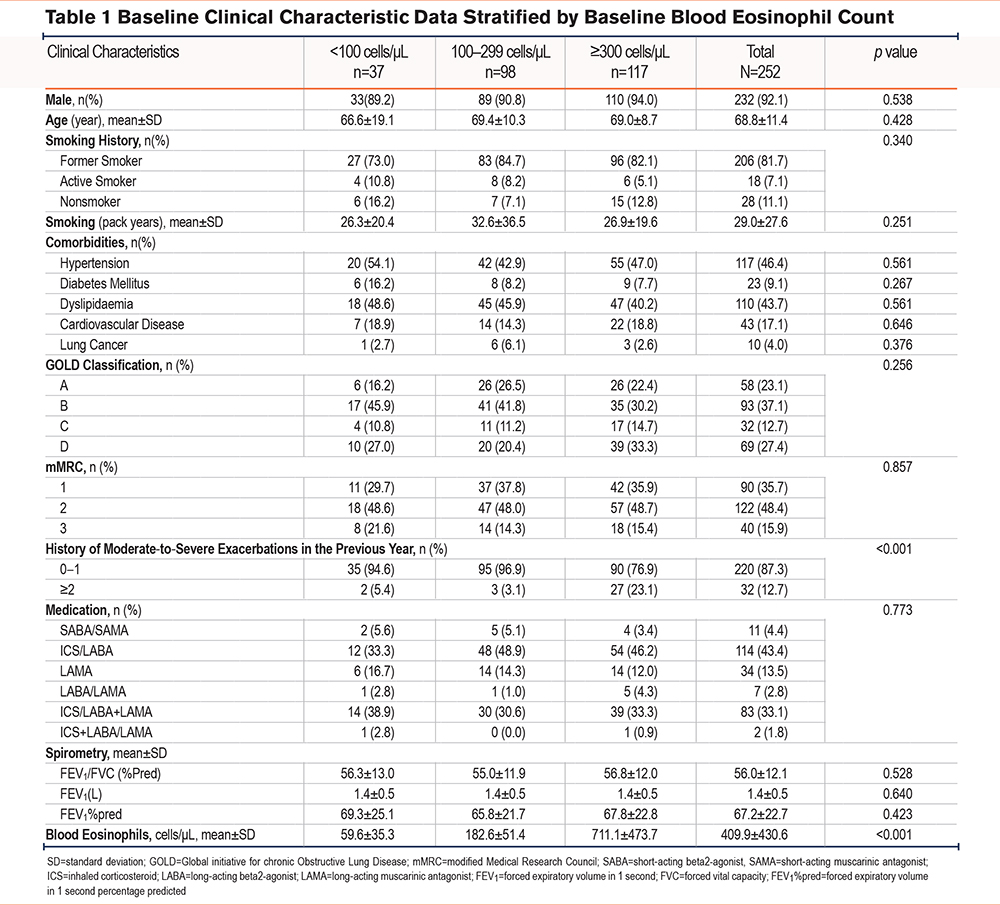
Patients were stratified into 3 groups according to their baseline BEC. There were 37 (14.7%), 98 (38.9%), and 117 (46.4%) patients in the <100, 100-299, and ≥300 cells/μL groups, respectively. There were no between-group differences in baseline characteristics.
The mean age was 68 years. Most patients with COPD were male. Most patients were former smokers, classified as GOLD B and D, and had an mMRC score of 2 or more. ICS/long-acting beta2-agonists (LABA) and ICS/LABA with inhaler triple therapy long-acting muscarinic antagonist (LAMA) were the 2 most common medications used in this population (Table 1).
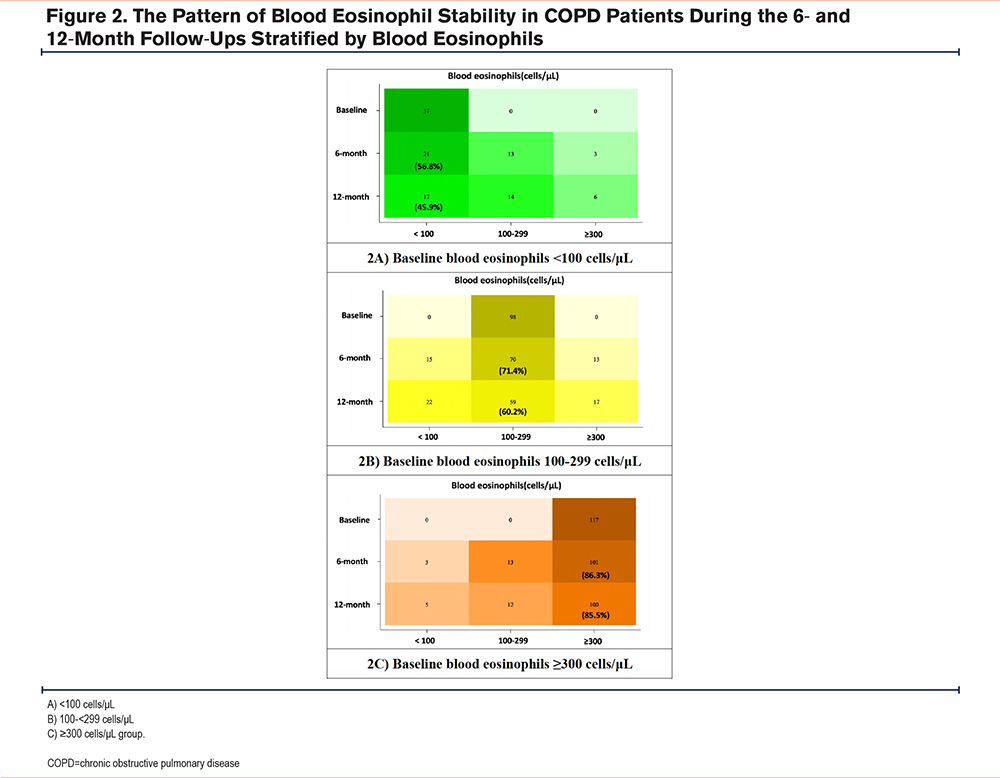
The Stability of Blood Eosinophil Counts
Overall, 75.9% and 66.3% of the patients had blood eosinophil counts in the same group at 6- and 12-months follow-up. The highest stability was observed in the blood eosinophils ≥300 cells/μL group (about 85%) at both 6 and 12 months. The 100-299 cells/μL group had a stability of 71% and 60% at 6 and 12 months, respectively. The lowest stability was observed in the <100 cells/μL group at only 57% and 46% at 6 and 12 months, respectively (Figure 2). The intraclass correlation coefficients (ICC) were 0.93, 0.74, and 0.88 in the BEC<100 cells/μL, 100-299 cells/μL, and ≥300 cells/μL groups, respectively after the 6- and 12-month follow-up periods, showing excellent correlation.
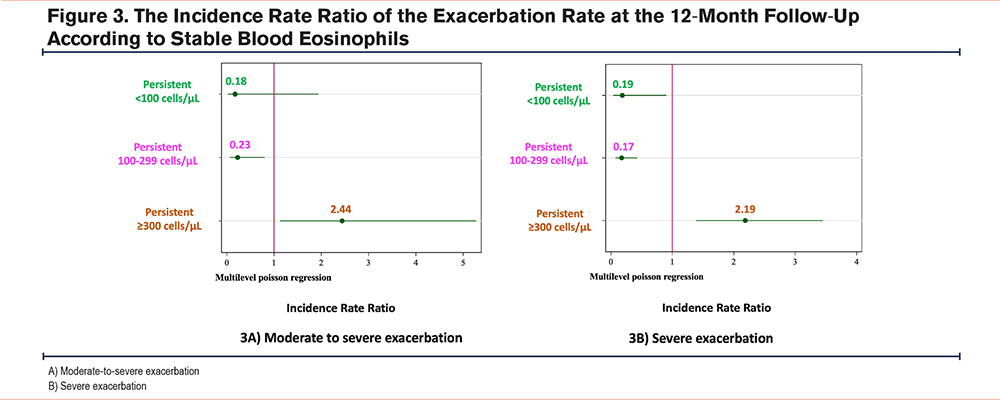
Clinical Outcomes
Moderate and Severe Exacerbation in Patients With COPD With Stable Blood Eosinophil Counts
All patients with COPD were followed for 12 months, and the association between exacerbation and the BEC in patients with stable blood eosinophil levels is shown in Figure 3. The persistent≥300 cells/μL group had a higher incidence of moderate-to-severe exacerbation (IRR 2.44, 95% confidence interval [CI]: 1.13-5.27, p value 0.023), as well as severe exacerbation (IRR 2.19, 95%CI: 1.39-3.45, p value 0.001). Both the persistent <100 cells/μL and 100-299 cells/μL groups had lower exacerbation rates than the ≥300 cells/μL group.
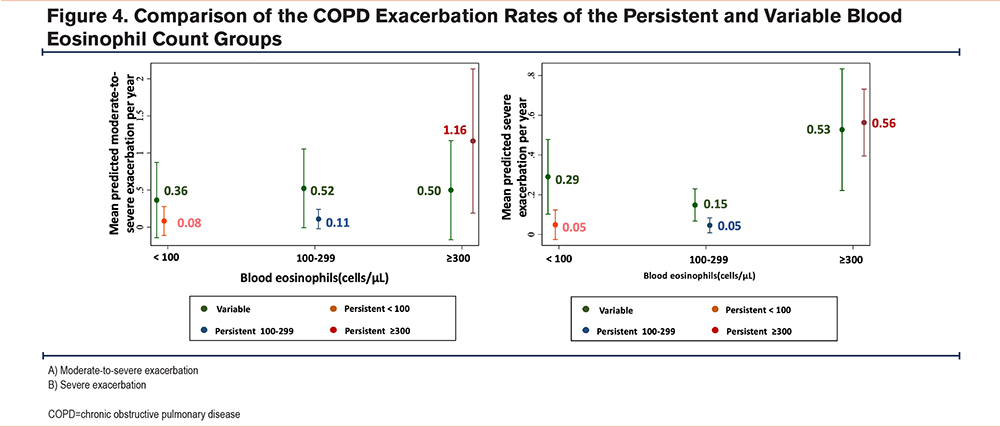
COPD Exacerbation Rates of the Persistent and Variable Blood Eosinophil Groups
The differences in moderate-to-severe exacerbations per year were compared for patients with COPD with persistent or variable blood eosinophil levels, as shown in Figure 4. The persistent BEC≥300 cells/μL group had apparent higher rates for both moderate-to-severe and severe exacerbation rates compared to the variable BEC≥300 cells/μL group. The rates of moderate-to-severe exacerbation were observed at 1.16 times per year (95%CI: 0.19-2.13, p value 0.019), while severe exacerbation occurred at a rate of 0.56 times per year (95%CI: 0.39-0.73, p value <0.001). Conversely, both the persistent <100 cells/μL and 100-299 cells/μL groups tended to have lower exacerbation rates than the variable groups.
Mortality Within the 12-Month Follow-Up
In terms of mortality, 4 (1.59%) patients died during the 12-month follow-up period. Two patients (5.13%) in the <100 cells/μL group died of pneumonia and acute myocardial infarction, whereas 2 (2%) in the 100-299 cells/μL group died of pneumonia and lung cancer. All patients received an ICS-containing regimen.
However, we were unable to identify a correlation between the stability of BEC and the rate of hospitalization or other patient-reported outcomes in this cohort.
Discussion
This study prospectively explored the stability of BEC in Thai patients with COPD at the 6- and 12-month follow-ups and examined the association between blood eosinophil stability, exacerbation rates, and other clinical outcomes.
This study shows that approximately half of Thai patients with COPD have a BEC≥300 cells/μL, which is higher than that in other populations. The overall stability of the BEC at 6 and 12 months was 75.9% and 66.3%, respectively. The highest stability (85%) was observed in the BEC≥300 cells/μL group, whereas the <100 cells/μL group had the lowest stability of approximately 50% after 12-months follow-up. Furthermore, the ICC in each BEC group during the study period showed excellent correlation. The persistent ≥300 cells/μL group had a higher incidence of moderate and/or severe exacerbation, than other blood eosinophil levels and the variable≥300 cells/μL group. Patients with COPD in the persistent <300 cells/μL group had lower exacerbation rates than those in the variable <300 cells/μL group.
This study revealed that 46.4% of patients with COPD exhibited a BEC of 300 cells/μL or higher. Before enrollment, patients with parasitic infections were identified and excluded through a combination of screening their medical histories and conducting stool examinations. The high BEC observed in our population is genuinely free from any interference caused by parasitic infections. This proportion of patients with a BEC≥300 cells/μL was notably greater than previous studies from various international countries.23-25 The observed discrepancy may be attributable to the distinctive characteristics of the Thai population. It is hypothesized that genetic predispositions and lifestyle factors play critical roles in this phenomenon. Furthermore, this study constituted a real-world, pragmatic clinical study in which a substantial cohort of patients with COPD was enrolled from a tertiary care hospital.
Our study indicates that patients with COPD exhibit high blood eosinophil stability, particularly those in the ≥300 cells/μL group; which has potential applications in clinical practice. This finding is similar to those of other studies that showed excellent correlation between repeated BECs in patients with COPD.12-14,26,27 Long et al12 found that the ICC of 12-month blood eosinophil stability was 0.84. The lowest stability was also observed in the <100 cells/μL group at only 47.4%, but the highest stability in this study12 was observed in patients with blood eosinophil levels of 100-299 cells/μL at approximately 77%. In the COPD cohort in the United Kingdom, approximately 65% of patients with COPD had persistently above or below 400 cells/μL, and the intraclass coefficient was 0.79 after 3-months follow-up.26 However, Southworth et al reported that the stability of blood eosinophils <150 cells/μL was quite stable at 87% and 86% after 6 months and >2 years follow-up.13 These results differed from those obtained in our study. We hypothesized that the different populations and natural histories of the patients with COPD are likely related to these results.
In our study, patients with COPD who had a BEC≥300 cells/μL had a very high stability; this could be used in clinical practice. Repeated BEC measurements were not required in this group. In the BEC<100 cells/μL group, the BEC increased over 12 months. The stability was only 46% after 12 months; therefore, a serial BEC is needed before identifying non-eosinophilic COPD and considering ICS withdrawal. In addition, in the BEC 100-299 cells/μL group, blood eosinophil levels were more variable. Follow-up BECs should also be performed, and the clinical risk of exacerbation should be considered with the BEC in guided ICS-based therapy. However, the <100 cells/μL and ≥300 cells/μL thresholds are estimates and are not to be considered cut-off values for treatment planning, as mentioned in the GOLD guidelines.21 This is the most important aspect that should be considered by clinicians.
According to existing research, the BEC is a significant predictive factor of COPD exacerbation.6,25,28,29 In this study we analyzed the association between blood eosinophil stability and exacerbation rates; the consistent ≥300 cells/μL group had a higher incidence of moderate-to-severe exacerbation. The incidence rate ratio of exacerbation was quite low in our study compared with other studies10,25,28,29; because this study was conducted in a tertiary setting, our patients were managed by pulmonologists and internists.
ICS/LABA was the most common medication prescribed in Thailand because of reimbursement, according to a previous Thai government policy, and LAMAs were approved a few years ago. This study will lead to proper management of COPD, especially in patients with COPD with a BEC<100 cells/μL.
To the best of our knowledge, this study is the first to prospectively examine blood eosinophil stability patterns in Thai patients with stable COPD, in an endemic area of parasitic infection. These findings support the role of blood eosinophils in the management of COPD, even among patients residing in an endemic area of parasitic infections. However, a limitation of this study is its single-center design. A multicenter study is needed to establish the stability of blood eosinophil levels in Thai patients with COPD.
Conclusion
Blood eosinophil levels showed good stability in patients with COPD with BEC≥300 cells/µL after 6- and 12-month follow-ups. Conversely, blood eosinophil tests should be repeated in patients with COPD with a BEC<300 cells/µL due to their variability and should be incorporated with the risk of exacerbation.
Acknowledgements
Author contributions: PK was primarily responsible for the study design, data collection, data analysis, data interpretation, and manuscript preparation. SJ contributed to the study design, data interpretation, and manuscript preparation. AN contributed to the data collection, data interpretation, and manuscript preparation. SLG contributed to the data analysis and manuscript preparation. SD contributed to the data collection and manuscript preparation. All of the authors have read and approved the final version of the manuscript.
We gratefully thanks the English Language Editing Service from Editage for the English writing and proofing of this manuscript.
Data sharing statement: Data available on request from the authors.
Declaration of Interests
The authors declare that they have no competing interests.