Running Head: Physical Activity and Biomarkers in COPD
Funding Support: This work was supported in part by the Department of Veterans Affairs, Rehabilitation Research and Development Service (Career Development Award 2, F6847W [Moy]; CDA2 IK2RX002165 [Wan]; Merit O1150-R [Moy]) and Health Services and Research Development Service (Career Development Award 2, 21-187 [Robinson]).
Date of Acceptance: April 24, 2024 | Publication Online Date: May 3, 2024
Abbreviations: 6MWT=6-minute walk test; AEs=acute exacerbations; BMI=body mass index; CAD=coronary artery disease; CHF=congestive heart failure; CI=confidence interval; CKMM=muscle-type creatine kinase; COPD=chronic obstructive pulmonary disease; ELISA= enzyme-linked immunosorbent assay; ESC=Every Step Counts study; FEV1%pred=forced expiratory volume in 1 second percentage predicted; GOLD=Global initiative for chronic Obstructive Lung Disease; IQR=interquartile range; NT-proBNP=N-terminal pro-β-type natriuretic peptide; PA=physical activity; RAGE=receptor for advanced glycation end products; RCTs=randomized clinical trials; SAMO=improving exercise tolerance and health-related quality of life in Veterans with COPD study; SD=standard deviation; SE=standard error; sRAGE=soluble receptor for advanced glycation end products; VA=Veterans Affairs; WEB=Walking and Education to Breathe study
Citation: Berube MN, Robinson SA, Wan ES, Mongiardo MA, Finer EB, Moy ML. Physical activity and systemic biomarkers in persons with COPD: insights from a web-based pedometer-mediated intervention. Chronic Obstr Pulm Dis. 2024; 11(4): 369-381. doi: http://doi.org/10.15326/jcopdf.2023.0472
Online Supplemental Material: Read Online Supplemental Material (244KB)
Introduction
Chronic obstructive pulmonary disease (COPD) results in dyspnea and reductions in exercise performance.1 COPD is also associated with a wide range of systemic derangements that involve, to varying degrees, cardiovascular effects, systemic inflammation, and skeletal muscle dysfunction.1-4 Physical activity (PA) promotion is the standard of care and improves clinical outcomes for patients with COPD.1 Independent of lung function, increased PA is associated with decreased risk of hospitalization, acute exacerbations (AEs), and mortality.5-10 Currently, it is unclear whether community-based PA promotion impacts cardiac and skeletal muscle responses and whether changes in systemic inflammation mediate the observed benefits of PA promotion on morbidity and mortality in persons with COPD.
N-terminal pro-β-type natriuretic peptide (NT-proBNP), the soluble receptor for advanced glycation end products (sRAGE), and muscle-type creatine kinase (CKMM) show promise as potential biomarkers of systemic response to exercise and PA promotion. In COPD, NT-proBNP is a cardiac hormone that independently predicts PA,11 AEs,12 and mortality.13 A systemic biomarker for cardiac dysfunction, NT-proBNP levels increase as myocardial wall stress increases.14,15 It is plausible that dynamic hyperinflation or increases in pulmonary arterial pressure during exercise in COPD would negatively impact cardiac function and increase NT-proBNP levels. Secondly, although exercise training or PA promotion have well-known benefits in COPD, an anti-inflammatory effect has not yet been established.16-22 Most COPD studies have focused on only 1 or a limited number of markers of systemic inflammation and comparison between studies is limited by the heterogeneity of intensity, duration, frequency, and type of exercise studied.16-22 The soluble form of the pattern recognition receptor for advanced glycation end products (RAGE) is found in serum. RAGE contributes to the pathogenesis of COPD by inducing inflammation and tissue injury, while sRAGE counteracts these effects by acting as a decoy receptor and preventing ligands from binding to RAGE.23 Lower sRAGE levels are associated with greater cigarette smoking exposure and more severe emphysema.24,25 sRAGE has emerged as a functionally relevant marker of systemic inflammation across many clusters in COPD.26 Finally, changes in serum levels of CKMM, an indirect biomarker of skeletal muscle damage, have been studied after short, local, exhaustive exercise training in the laboratory in persons without27 and with COPD.28-31 It is unknown whether CKMM changes with community-based walking over longer periods of PA promotion.
The relationships between PA and exercise performance and NT-proBNP, sRAGE, and CKMM in persons with COPD are unclear. In addition, systemic biomarker responses to community-based PA promotion have not been well characterized in COPD. The current literature focuses on changes in these biomarkers in the laboratory setting after bouts of high-intensity exercise rather than community-based walking which more accurately reflects patients’ usual activities in their home environment. We have developed a web-based, pedometer-mediated PA intervention for persons with COPD32-35based on the Theory of Self-Regulation.36 The intervention provides 4 key components to promote behavior change: individualized goal setting, iterative step-count feedback for self-monitoring, educational and motivational content for disease self-management, and social support in the form of an online community forum. In 3 randomized controlled trials in persons with COPD, the intervention has been shown to be efficacious in increasing PA as measured by daily step counts, compared to controls, over 3–6 months.32-35 The PA intervention has been shown to be efficacious in improving daily step counts by 779, 804, and 1312 steps per day,33-35 compared to controls. These improvements are both clinically and statistically significant based on published minimal clinically important differences for daily step count that range from 350 to 1100 steps per day37,38and their association with a decreased risk for AEs.39
In this secondary data analysis, we explored the baseline cross-sectional associations between PA and exercise performance with NT-proBNP, sRAGE, and CKMM. We hypothesized that higher NT-proBNP, CKMM, and sRAGE would be associated with greater PA/exercise. As a separate goal, we explored whether the magnitude of changes in daily step counts resulting from a 3-month PA intervention that promotes community-based walking would impact these biomarkers. We hypothesized that a 3-month walking intervention would not change NT-proBNP or CKMM, and may decrease systemic inflammation as reflected by an increase in sRAGE.
Methods
Participants and Characteristics
This secondary analysis uses data and blood collected from 3 previously published PA studies: an improving exercise tolerance and health-related quality of life in Veterans with COPD study known as SAMO,40 the Every Step Counts study (ESC),35and the Walking and Education to Breathe study (WEB).34 SAMO was an observational study of daily step counts that enrolled participants with stable COPD from January 2009 to February 2011.40 ESC and WEB were randomized controlled trials examining the efficacy of a web-based, pedometer-mediated intervention to increase PA compared to pedometer alone or a control group, respectively.34,35 Participants were enrolled in ESC35 from April 2012 to August 2015. They were randomized to 1 of 2 groups for 3 months: Omron HJ-720 ITC pedometer and a web-based PA intervention or Omron alone with written materials about exercise (i.e., control).35 Participants were enrolled in WEB from May 2015 to February 2019 from 2 Veterans Affairs (VA) Medical Centers34; the current analyses use data from only one VA Medical Center. WEB participants were randomly assigned to either a Fitbit Zip pedometer and the same web-based PA intervention or usual care and written instructions for walking and exercise (i.e., control) for 6 months.34 For all 3 studies, data from assessments and blood draws conducted at baseline and 3 months were used in this secondary analysis. Detailed information about these 3 studies is published elsewhere.34,35,40
In all 3 studies, at baseline, forced expiratory volume in 1 (FEV1) second was measured using an Eaglet spirometer (nSpire Health, Inc., Longmont, Colorado).41 Body mass index (BMI) was calculated after measuring participants’ height and weight. Using self-report and medical chart review, we ascertained age, sex, race, pack years, current supplemental oxygen use, history of coronary artery disease (CAD), history of congestive heart failure (CHF), and occurrence of AEs in the year prior to study entry.
Systemic Biomarkers
At the baseline and 3-month visits, peripheral blood was collected by venipuncture into vacutainer tubes with ethylenediaminetetraacetic acid anticoagulant. Blood was collected between 9:00 AM and 4:00 PM. Plasma was obtained by centrifugation at 1459 × g for 15 min. The samples were stored at -80 degrees Celsius in liquid nitrogen from the time they were drawn in the parent studies until the assays were performed in July 2021. There were no repeated freeze-thaw cycles. Plasma NT-proBNP, sRAGE, and CKMM levels were measured by the Clinical and Epidemiologic Research Laboratory, Children’s Hospital, Boston, Massachusetts. NT-proBNP levels were determined using an electrochemiluminescent quantitative sandwich enzyme immunoassay technique on the Roche Cobas 6000 system (Roche Diagnostics, Indianapolis, Indiana). This immunoassay technique has a sensitivity of 5pg/mL and day-to-day imprecision values at concentrations of 46, 125, and 32,805pg/mL of 3.1%, 2.7%, and 2.7%, respectively. sRAGE levels were determined using an enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Minneapolis, Minnesota), specifically an enzymatically amplified "2-step" sandwich-type immunoassay. This ELISA assay detected any form of soluble RAGE, including sRAGE, endogenous secretory RAGE, and matrix metalloproteinase-cleaved membrane-bound RAGE. This assay has a sensitivity of 4.12pg/mL, and day-to-day variabilities of the assay at concentrations of 519, 1449, and 2890pg/mL are 8.2%, 8.2%, and 6.6% respectively. Lastly, CKMM levels were determined using an ELISA assay (Novus Biologicals, Centennial, Colorado) that uses the Sandwich-ELISA principle. This ELISA assay has a sensitivity of 0.94ng/mL and day-to-day variabilities of the assay at concentrations of 257.1 and 500.8ng/mL are 11.6% and 10.8%, respectively.
Physical Activity and Exercise Performance
In the 3 studies, PA was directly measured as daily step count at baseline and 3 months. In SAMO, daily step counts were captured using the Omron pedometer and monitored over 14 days at baseline and 3 months.40 In ESC, participants also wore the Omron pedometer; daily step counts were monitored over 7 days at baseline and 3 months.35 In WEB, participants used the Fitbit Zip pedometer; daily step counts were monitored over 10 days at baseline and over 14 days at 3 months.34 The Omron pedometer and the Fitbit Zip pedometer, both worn on the waist, accurately measure daily step counts in people with COPD.42,43 Exercise performance was assessed by the 6-minute walk test (6MWT) at baseline and 3 months. The 6MWT was performed following American Thoracic Society guidelines, except that a practice test was not performed.44
Statistical Analysis
All analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina). Univariate analyses examined baseline sample characteristics: t-tests and analyses of variances assessed the association of systemic biomarker levels with categorical variables. Pearson correlations examined associations between biomarker levels and continuous variables. For the models examining whether the PA intervention impacted systemic biomarker levels, we maintained the randomization group assignments. Since no SAMO participants received the web-based PA intervention, they were considered controls. General linear models (PROC GLM) explored associations between: (1) baseline daily step count and 6MWT distance and baseline level of each systemic biomarker (NT-proBNP, sRAGE and CKMM), and (2) the effect of the PA intervention on change in systemic biomarkers, compared to controls.
Since systemic biomarker distributions were skewed, each value was natural log-transformed to meet model assumptions. Change from baseline to 3 months for all biomarkers was calculated after the natural log-transformation, i.e., ln(3 months) – ln(baseline). For ease of interpretation of results, we calculated the concentration levels of each systemic biomarker by exponentiating the natural log-transformed estimates using a base of e. Clinical and demographic characteristics that were significantly associated with baseline levels of biomarkers were included as covariates. CAD and CHF were included in the main models because they are not necessarily interchangeable and CHF is an essential comorbidity when studying NT-proBNP. Final models adjusted for study, age, race, sex, FEV1 percentage predicted (%pred), BMI, CAD, CHF, oxygen use, and history of AEs in the year prior to study entry. Statistical significance was defined as p<0.05.
Results
Participant Characteristics
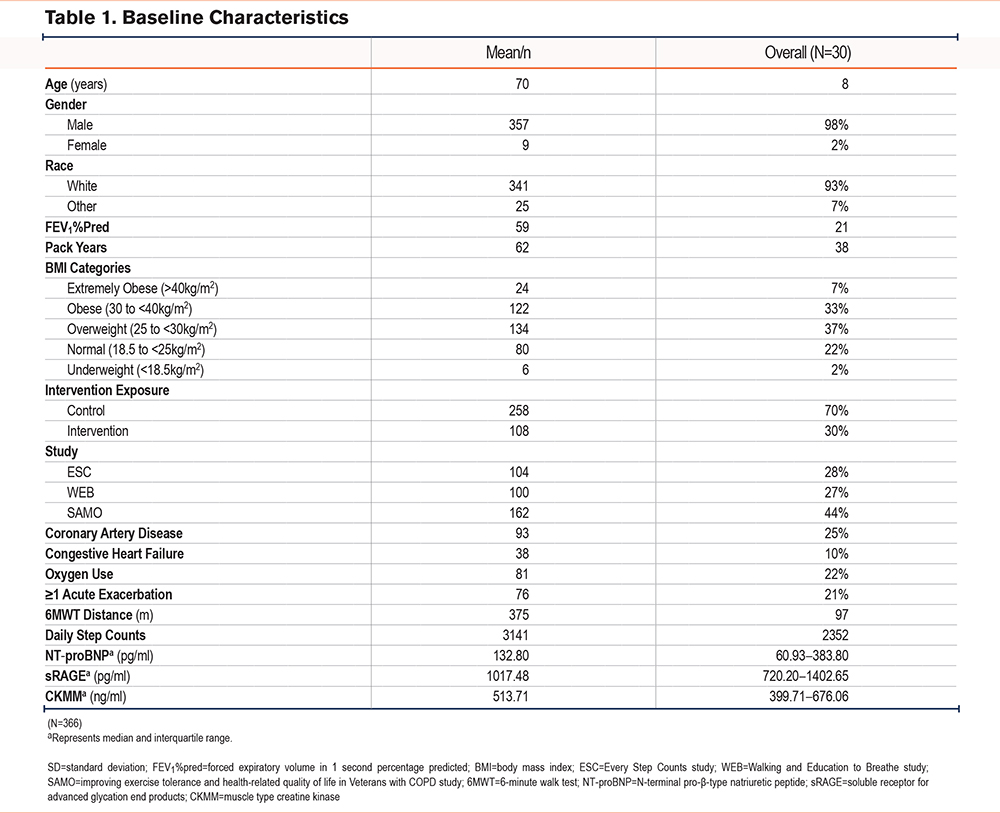
Of the 375 participants enrolled in the 3 studies, 366 had valid step-count data, performed the 6MWT, and provided blood samples at baseline. Most participants were White (93%, n=341) and males (98%, n=357) with mean age 70±8 years (Table 1). The mean daily step count was 3141±2352 and mean 6MWT distance was 375±97m. Median NT-proBNP serum level was 132.80pg/ml (interquartile range [IQR]=60.93–383.80), median sRAGE serum level was 1017.48pg/ml (IQR=720.20–1402.65), and median CKMM serum level was 513.71ng/ml (IQR=399.71–676.06).
Higher Global initiative for chronic Obstructive Lung Disease (GOLD)45 stage (i.e., lower FEV1%pred) was significantly associated with lower sRAGE, but not NT-proBNP or CKMM. Increased BMI was significantly associated with higher average CKMM (Supplemental Table E1 in the online supplement). Cigarette smoking history measured by pack years was not associated with systemic biomarker levels (Supplemental Table E2 in the online supplement). Participants with a history of CAD had higher mean levels of NT-proBNP and sRAGE compared to those without CAD. Participants with a history of CHF had higher mean levels of all 3 biomarkers, compared to those without CHF. Those using supplemental oxygen had higher mean NT-proBNP and CKMM levels than those not on oxygen. There was no difference in the 3 systemic biomarkers among those with at least one AE compared to those with no AE in the year prior to study entry. Compared to participants in SAMO, those in ESC and WEB had significantly lower levels of NT-proBNP. Finally, there was no difference in systemic biomarker levels between those in the intervention versus control groups (Supplemental Table E1 in the online supplement).
Baseline Relationships
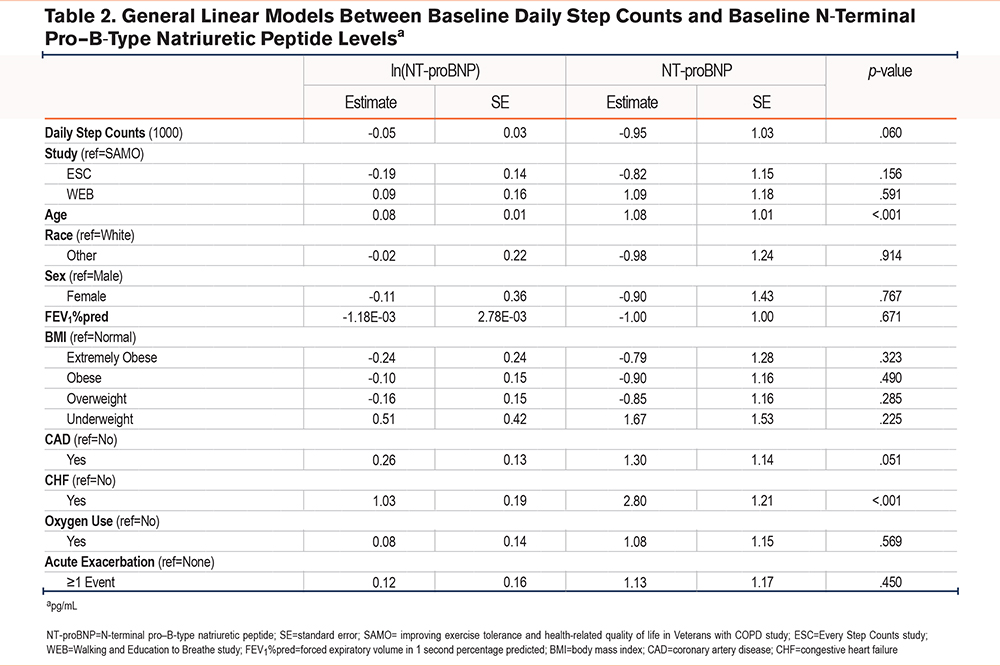
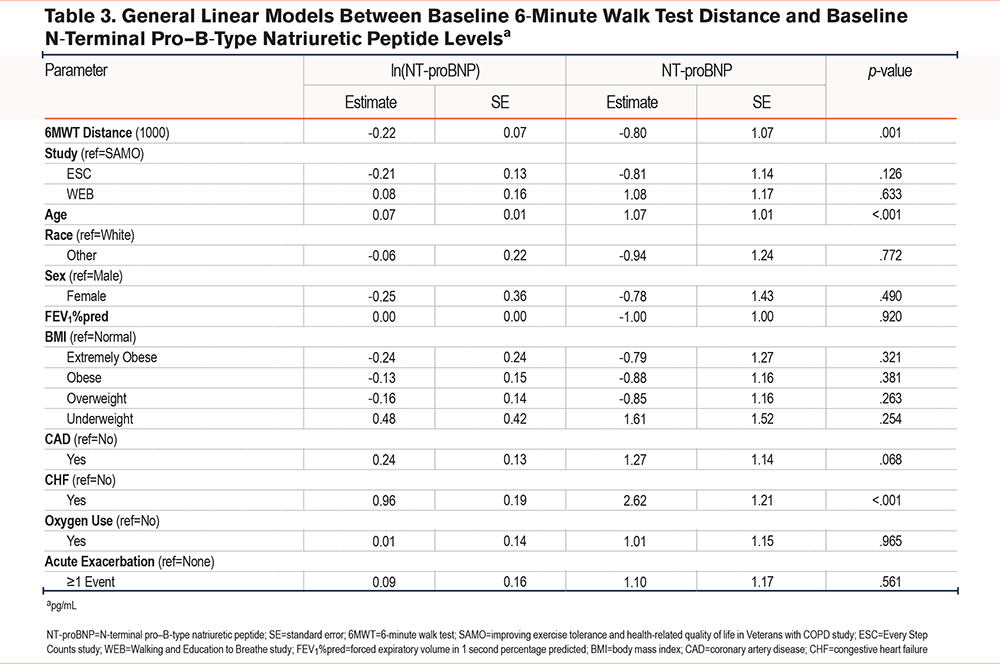
At baseline, adjusted models showed an association between higher daily step counts and lower NT-proBNP levels (β=-0.95pg/ml per 1000 steps per day, 95% confidence interval [CI] [-0.91, 1.00], p=.060; Table 2). Baseline daily step counts were not associated with sRAGE (Supplemental Table E3 in the online supplement) or CKMM levels (Supplemental Table E4 in the online supplement). There was a significant association between higher 6MWT distance and lower NT-proBNP levels (β=-0.80pg/ml per 100 meters, 95% CI [-0.71, -0.92], p=.001) (Table 3). Baseline 6MWT distance was not significantly associated with sRAGE (Supplemental Table E5 in the online supplement) or CKMM (Supplemental Table E6 in the online supplement) levels.
Compared to participants in SAMO, those in the ESC study had significantly higher levels of sRAGE and those in the WEB study had significantly lower levels of CKMM. Age was consistently an independent predictor of NT-proBNP, sRAGE, and CKMM levels, such that the greater the age, the higher the systemic biomarker levels. Lower FEV1%pred was significantly associated with lower sRAGE levels. Compared to those with normal BMI, those who were underweight had significantly lower CKMM levels, while those who were overweight or obese had significantly higher levels of CKMM. Compared to those with no history of CAD, participants with CAD had NT-proBNP concentrations that were 1.27 (p=.068) to 1.30 (p=.051) pg/ml higher. Finally, compared to those with no history of CHF, participants with CHF had NT-proBNP concentrations that were 2.62 to 2.80pg/ml higher (p≤.001).
Effect of the PA Intervention
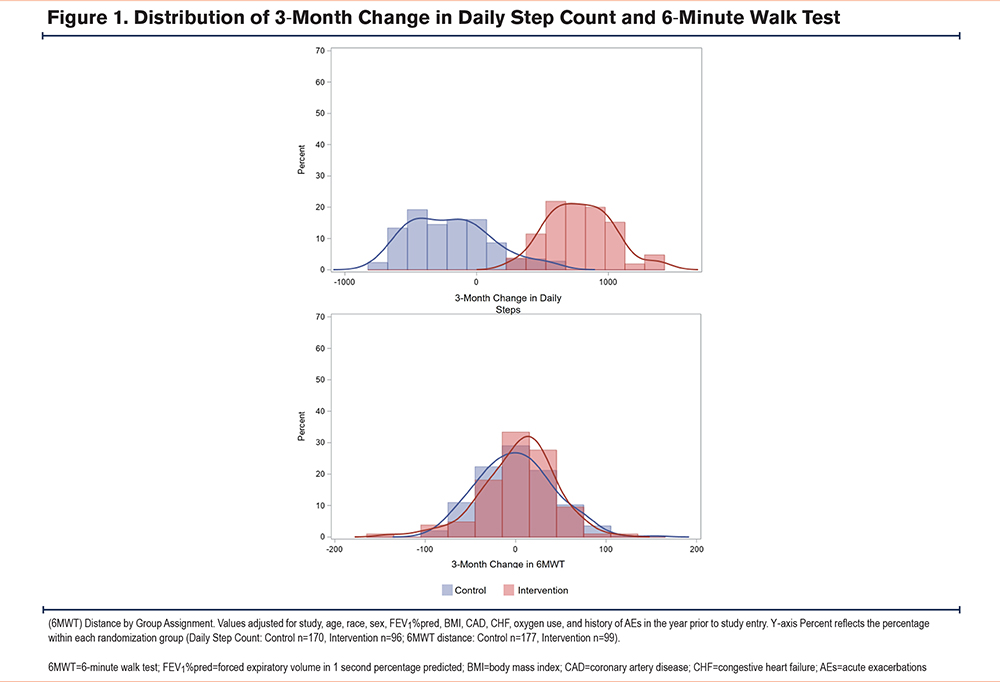
By randomization group, the mean 3-month change in daily step counts, adjusted for covariates, was significantly greater in the intervention group (789±1864) compared to the control group (-174±1448; β=801.61, 95% CI [295, 1308], p=.002; Figure 1, top panel). There was no significant difference in mean 3-month change in 6MWT distance between the intervention group and control group (2.14±157.91m versus 0.37 ±146.81m, β=1.75, 95% CI [-43.43, 46.92], p=.939; Figure 1, bottom panel). There was no significant effect of randomization group on change in levels of NT-proBNP, sRAGE, or CKMM, compared to the control group (Figure 2).
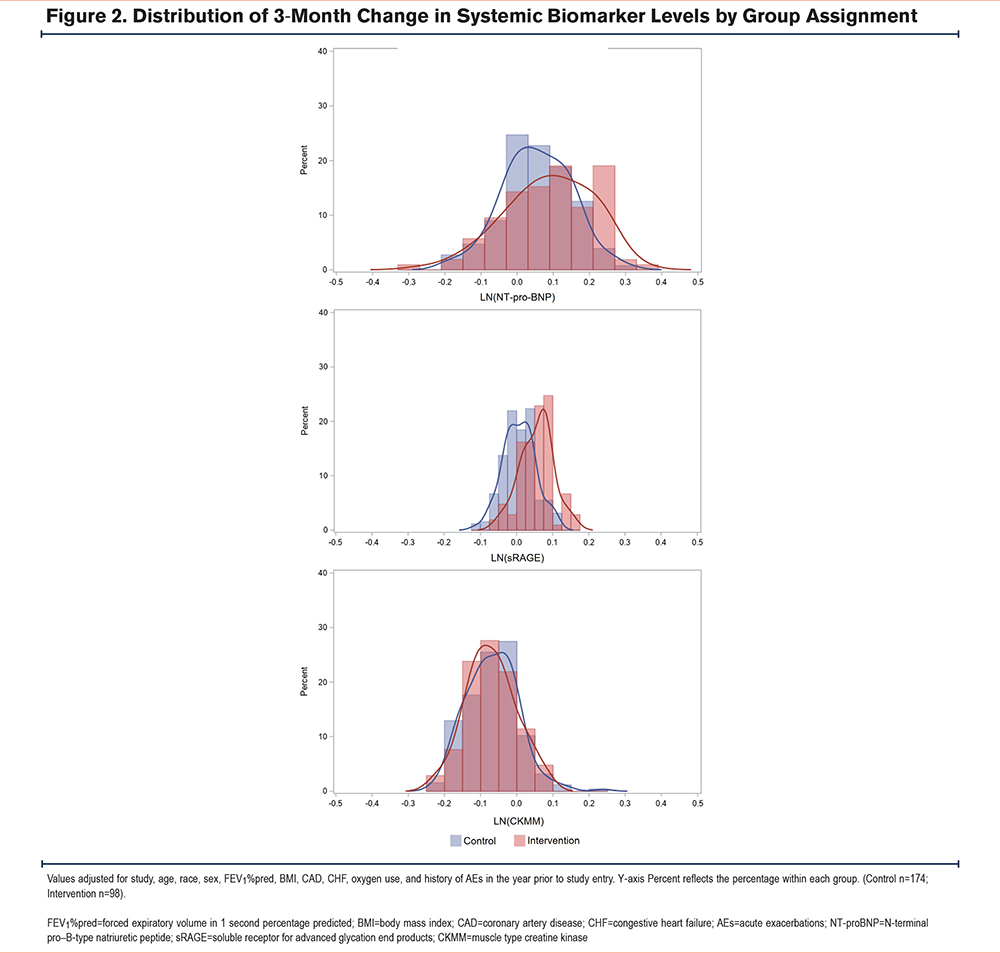
Discussion
In this secondary, exploratory data analysis, we show that at baseline, cross-sectionally higher exercise performance is statistically associated with lower NT-proBNP levels; there was no relationship between PA and exercise performance with sRAGE and CKMM. Additionally, we believe that for the first time, we show that a 3-month PA intervention promoting community-based walking has no negative impacts on myocardial stress or muscle injury as assessed by NT-proBNP and CKMM, respectively. Physical activity promotion over 3 months also has no effect on systemic inflammation measured by sRAGE. Our preliminary findings suggest that PA promotion in the form of community-based walking over 3 months (as opposed to bouts of high-intensity exercise training in the laboratory) does not impact biomarker levels that would suggest cardiac dysfunction, skeletal muscle damage, or inflammation. These results may guide the personalization of PA interventions by helping to identify who can tolerate increases in exercise intensity and duration (if no change in blood biomarkers) and who requires decreases in intensity (if increase in blood biomarkers).
History of CAD and CHF were independent predictors of higher NT-proBNP levels in this cohort of participants with COPD, similar to that shown in the general population.46 We showed that higher exercise performance in persons with COPD is significantly associated with lower NT-proBNP levels, likely reflecting a complex interaction between COPD disease severity and cardiovascular comorbidities such as CAD.3 Secretion of NT-proBNP increases as myocardial wall stress increases and is a systemic biomarker for heart failure.47 It is plausible that PA or exercise in persons with COPD is associated with dynamic hyperinflation or elevated pulmonary arterial pressure that may negatively impact cardiac function, with accompanying higher NT-proBNP levels.47-49 However, we showed no negative impact of our 3-month PA intervention on myocardial stress and no relationship between change in daily step count or change in 6MWT distance with change in NT-proBNP. These results suggest that the magnitude of changes in daily step count and 6MWT distance observed over 3 months, which spanned a wide range, do not negatively impact cardiac function.
More recently, there has been interest in sRAGE which has emerged as an inflammatory biomarker common across several clusters in COPD.26 We showed that there is no significant association between daily step counts or 6MWT distance and sRAGE. We also showed that lower FEV1%pred (i.e., greater COPD disease severity) is significantly associated with lower sRAGE (i.e., greater systemic inflammation) which is consistent with published findings.23,24 Although exercise training, pulmonary rehabilitation, and PA promotion have demonstrated benefits in COPD, an anti-inflammatory effect of exercise has not yet been established.16-22 Most COPD studies, heterogeneous with respect to intensity, duration, frequency, and type of exercise studied, have focused on only 1 or a limited number of biomarkers of systemic inflammation.16-22 We have previously shown cross-sectionally that greater PA as measured by daily step counts is associated with lower plasma levels of C-reactive protein and interleukin-6, two inflammatory biomarkers in COPD.50,51
There is conflicting literature on the impact of exercise training on biomarkers of systemic inflammation. The current results do not show an impact of our web-based, pedometer-mediated promotion of community-based walking specifically on sRAGE. It is plausible that more strenuous exercise is needed to impact expression of sRAGE. In addition, 3 months may have been insufficient time to effect changes on sRAGE levels. There is one published study52 examining community-based PA in persons with COPD as promoted with a cell phone intervention that showed decreases in serum C-reactive protein and interleukin-8. In the general population, a few studies have shown conflicting results in terms of the effects of exercise training and PA promotion on sRAGE.53-55 Army recruits engaged in a 4-week exercise intervention had decreases in sRAGE53 and community volunteers in Japan enrolled in a 6-month PA promotion program had decreases in sRAGE,54 while a study of adults aged 30–65 with at least one cardiovascular risk factor engaged in an 8-month PA program had an increase in sRAGE.55
Finally, decreased PA in persons with COPD and the resultant progressive muscle deconditioning may lead to disuse atrophy.4 A common symptom of COPD is “muscle wasting,” in which several factors, including disuse and hypoxemia, lead to muscle atrophy.49 We showed no significant cross-sectional associations between PA and exercise performance with CKMM. Since previous studies have examined short, exhaustive exercise training in the laboratory, it is unclear what effects longer-term PA promotion to counter deconditioning may have on skeletal muscles and CKMM levels. We demonstrated no changes in CKMM in response to our PA intervention. Our results suggest that community-based walking in persons with COPD over a 3-month timeframe, resulting in the magnitude of change in daily step count and 6MWT distance we observed, does not damage skeletal muscles as reflected in the lack of change in CKMM levels. Previous small studies of short, exhaustive exercise training in the laboratory in persons with COPD have demonstrated mixed results with increased serum levels of creatine kinase, including CKMM, while others have shown no change in CK levels.27-31 Interestingly, BMI is an independent predictor of CKMM, with those who are overweight or obese having higher CKMM levels and those who are underweight having lower CKMM levels, compared to those with a normal BMI.
It is possible that we observed no changes in the biomarkers because the intensity and duration of our intervention were not sufficient to elicit changes. We see it as a good thing that there is no biomarker evidence for myocardial stress or muscle injury after a 3-month community-based walking PA intervention that has been shown to result in increases in daily step counts which are statistically and clinically significant. In the parent randomized clinical trials (RCTs), the PA intervention has been shown to be efficacious in improving daily step counts by 779–1312 steps per day, compared to controls.33-35 These improvements are both clinically and statistically significant based on published minimal clinically important differences for daily step count.37,38 Importantly, we showed that this modest increase in daily step counts in this frail population is associated with a reduction in risk for acute exacerbations.39 For these clinically significant and statistically significant observed changes in physical activity, we did not see any changes in the biomarkers. Our goal was not to develop an intervention of such intensity or duration that there would be deleterious impacts on the heart and skeletal muscles to see increases in NT-proBNP and CKMM.
While participants in the intervention groups in ESC and WEB, on average, improved their daily step count more than the control groups, there were participants in both randomization groups who experienced improvements from baseline. In analyses combining the groups and examining baseline and 3-month change values of daily step count and 6MWT distance and levels of systemic biomarkers, independent of group assignment, the results were similar to what we report. In our cross-sectional analyses, we presented the model estimates per 1000 steps per day and per 100 meters for the 6MWT to view the numbers most easily. However, the clinical implications of these estimates remain unclear as these results are cross-sectional associations, precluding any conclusions about causal changes in NT-proBNP.
This secondary data analysis has many strengths including the well-characterized cohort of participants with COPD, a PA intervention with proven efficacy, and the ability to examine change in PA/exercise performance and change in systemic biomarker levels across 3 months in a large sample. The biomarkers examined represent a broad spectrum of systemic responses to PA and exercise. Our novel finding that a PA intervention promoting community-based walking has no deleterious effects on myocardial stress, systemic inflammation, and muscle injury assessed by NT-proBNP, sRAGE, and CKMM, respectively, is useful in guiding clinical care. Although we did not observe significant relationships between changes in PA/exercise performance and changes in systemic biomarker levels, these results are important to inform how best to monitor systemic response to PA promotion in COPD.
We were limited in the exploratory nature of this data analysis that combined 3 separate studies with significant differences in sRAGE and CKMM between studies. Nevertheless, all models adjusted for study to account for these differences. We acknowledge the missing follow-up data and that the results reflect only the subset of participants who participated in follow-up. The demographics of the populations from each study were homogeneous, with most participants being White, male, and U.S. Veterans, limiting the generalizability of these results to other populations. The follow-up time of 3 months was relatively short and may not have allowed enough time for changes in serum biomarker levels to occur. We acknowledge that it is unknown whether the biomarker levels assessed at 3 months reflect PA/exercise over the preceding 3 months or merely the preceding 3 hours or 3 days. However, it is certainly a different time point compared to the measurement collected at baseline since participants have been engaged in a 3-month PA intervention with ongoing community-based walking. There is very limited literature examining the long-term stability of these biomarkers. However, there is precedence for analysis of samples that have undergone long-term storage. For example, 3 NHANES studies56-58 published in 2023 examined NT-proBNP in samples collected between 1999–2004 while the assays were performed 19–21 years later between 2018–2020. We emphasize that we are not presenting absolute values as thresholds for clinical decision-making. If there is decay, the decline in values would affect both groups with no preferential bias for one group over another. The cohort included only 4 current smokers precluding any informative further subgroup analyses on smoking status. Although the parent RCTs would have balanced unmeasured confounders between groups, it is possible that variables such as chronic kidney disease and diuretic use may impact the results of our models. These relationships should be further explored in future studies, tracking systemic biomarker levels over longer periods of time with interventions of varying levels of exercise intensity to better understand systemic responses to PA promotion in persons with COPD.
Conclusions
Higher 6MWT distance is associated with lower NT-proBNP levels. A 3-month PA intervention promoting community-based walking has no negative impacts on myocardial stress or muscle injury as assessed by NT-proBNP and CKMM, respectively. It also has no effect on systemic inflammation measured by sRAGE. These results may help personalize PA interventions and identify who can tolerate increases in exercise intensity and duration. Further studies are needed to understand the systemic biomarker responses to PA and exercise promotion in persons with COPD.
Acknowledgements
Author contributions: All authors contributed to the conception of the work, the analysis and interpretation of data for the work, drafting and revising the work critically for important intellectual content, and final approval of the version submitted for publication.
Declaration of Interests
The authors have no conflicts of interest to declare.