Running Head: COPD With Lung Cancer in Older Adults
Funding Support: Funding was provided by the National Cancer Institute (R01-CA-264176) and the National Institute of Environmental Health Sciences (2T32ES007018-46).
Date of Acceptance: May 24, 2024 | Publication Online Date: June 5, 2024
Abbreviations: AECOPD=acute exacerbation of COPD; AIAN=American Indian/Alaskan Native; CCI=Charlson Comorbidity Score Index; CI=confidence interval; CLD=confidence limit difference; CLR=confidence limit ratio; COPD=chronic obstructive pulmonary disease; DID=difference in prevalence differences; FFS=fee-for-service; ICD=International Classification of Diseases; LCL=lower confidence limit; NHB=non-Hispanic Black; NHPI=Native Hawaiian or Pacific Islander; NHW=non-Hispanic White; NSCLC=non-small cell lung cancer; PD=prevalence difference; PR=prevalence ratio; RPR=ratio of prevalence ratios; SCLC=small cell lung cancer; SD=standard deviation; SEER=Surveillance, Epidemiology, and End Results; SES=socioeconomic status; UCL=upper confidence limit
Citation: Metwally EM, Lund JL, Drummond MB, Peacock Hinton S, Poole C, Thompson CA. COPD with lung cancer among older United States adults: prevalence, diagnostic timeliness, and association with earlier stage tumors. Chronic Obstr Pulm Dis. 2024; 11(4): 382-395. doi: http://doi.org/10.15326/jcopdf.2024.0489
Online Supplemental Material: Read Online Supplemental Material (657KB)
Introduction
Lung cancer is the most common cause of cancer-related death worldwide.1 The 5-year survival of lung cancer ranges between 62% for early-stage disease to 8% for late-stage, with important disparities among racial minorities in the United States.1 Survival from lung cancer depends on tumor type, access to care, and preexisting comorbidities.2,3 Chronic obstructive pulmonary disease (COPD) is a common comorbidity among patients with lung cancer and an independent risk factor for lung cancer regardless of smoking history.4 Previous studies reported that the annual incidence of lung cancer is ~5 times higher among patients with COPD compared to the general population.5 Further, severe COPD is a competing cause of death among patients with lung cancer.6 Both diseases are frequently diagnosed among older adult populations when patients are more likely to be frail, impacting their treatment tolerance, quality of life, and survival.7 Therefore, several studies8-11 have emphasized the importance of including COPD in risk prediction models to improve ongoing efforts for diagnosis of lung cancer at an early stage where curable treatment is available, and quality of life as well as survival are boosted.
However, COPD is frequently underdiagnosed in the general population, among participants in lung cancer screenings, and patients with new lung cancer diagnoses.12,13 Reports from previous studies showed that underdiagnosis of COPD is higher among populations at high risk to develop and die from lung cancer such as racial minorities and populations with a low socioeconomic status (SES) in the United States.14,15 Non-Hispanic Black (NHB) populations are less likely to have a COPD diagnosis regardless of the severity of airflow obstruction,16 and more likely to develop lung cancer at younger ages and less smoking intensity compared to non-Hispanic White (NHWs) populations.17 Low SES has been shown to be associated with a greater risk of both COPD and lung cancer.18
Little is known about prevalence and timing of COPD diagnoses among patients with lung cancer and across different racial and SES backgrounds in the United States. Further, there is lack of evidence about whether a preexisting COPD diagnosis is associated with earlier-stage lung cancer at diagnosis.19 Therefore, in the present study we sought to estimate the prevalence of a COPD diagnosis among patients diagnosed with lung cancer, the timing of a COPD diagnosis relative to the start of their lung cancer diagnostic episode, and, among those with both COPD and lung cancer, the association between timing of a COPD diagnosis and lung cancer stage at diagnosis.
Methods
Data Source
We used the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) linked to Medicare fee-for-service (FFS) insurance claims data from the Centers for Medicaid and Medicare Services. The SEER-Medicare data reflects linkage of 2 large population-based sources of data to provide detailed information about Medicare beneficiaries with cancer. We used the SEER database which includes data from 21 cancer registries and represents approximately 40% of the U.S. population.20 The SEER tumor registry file includes patient sociodemographic and tumor characteristics. The Medicare claims file includes all details of reimbursed health care services that occurred in different settings (e.g., hospitals, physician offices, outpatient clinics) from the time of the patient’s Medicare enrollment until disenrollment or death.
Study Population
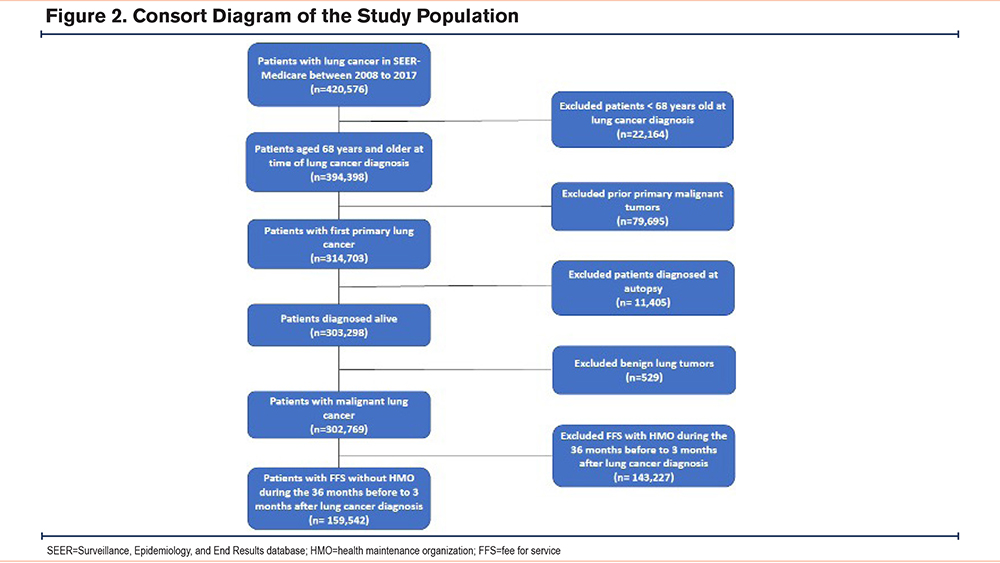
The study population included Medicare beneficiaries who were diagnosed with primary invasive lung cancer between 2008 to 2017. To ensure we captured all relevant prediagnostic health care utilization in the Medicare FFS claims, patients were included if they had continuous enrollment in Medicare Parts A and B without health maintenance organization coverage in the 36 months before diagnosis until 3 months after lung cancer diagnosis (or until death). Given the typical start of Medicare coverage at age 65 years in the United States and our requirement of 3 years of continuous enrollment to reduce COPD misclassification,21 we included patients 68 years of age and older. We excluded patients who were diagnosed at autopsy/death certificate, had lung cancer staged in situ, had a previous history of cancer, or were younger than 68 years old at diagnosis (Supplementary Figure E1 in the online supplement).
Index Date
To characterize the health care episode in which the lung cancer was first detected, we used a published and validated algorithm22 that identifies the earliest International Classification of Diseases (ICD)-9th and 10 Revisions claim code associated with lung cancer within +/- 1 month of the SEER diagnosis month. This “index date” approximates the start of the cancer care episode, i.e., when care delivery for the suspected lung cancer began. If no Medicare claims were found within +/- 1 month of the SEER diagnosis month, the index date was imputed as the 15th day of the SEER month.
COPD Diagnosis and Timing
We used a validated claims-based algorithm23 to identify the occurrence of 1 or more COPD diagnosis codes (ICD-9 491, 491.2, 492, 496 and ICD-10 J40, J41, J43.0, J43.1, J43.2, J43.8, J43.9, J44) from all Medicare claims in the 36 months before, to 3 months after, the index date. We further classified COPD based on its timing relative to the lung cancer diagnostic episode. Patients whose earliest COPD-related claims occurred during the lung cancer peri-diagnosis period, -3 to +3 months around the index date, were classified as having “concurrent COPD”; all others were classified as having “preexisting COPD”. This framing reflects our hypothesis, that some patients might not receive their COPD diagnosis until after the start of the lung cancer diagnostic episode, i.e., during pretreatment evaluation of lung cancer (e.g., pulmonary function testing). It also reflects precedent for defining the “peri-diagnosis” period in health care utilization data24,25(Figure 1).
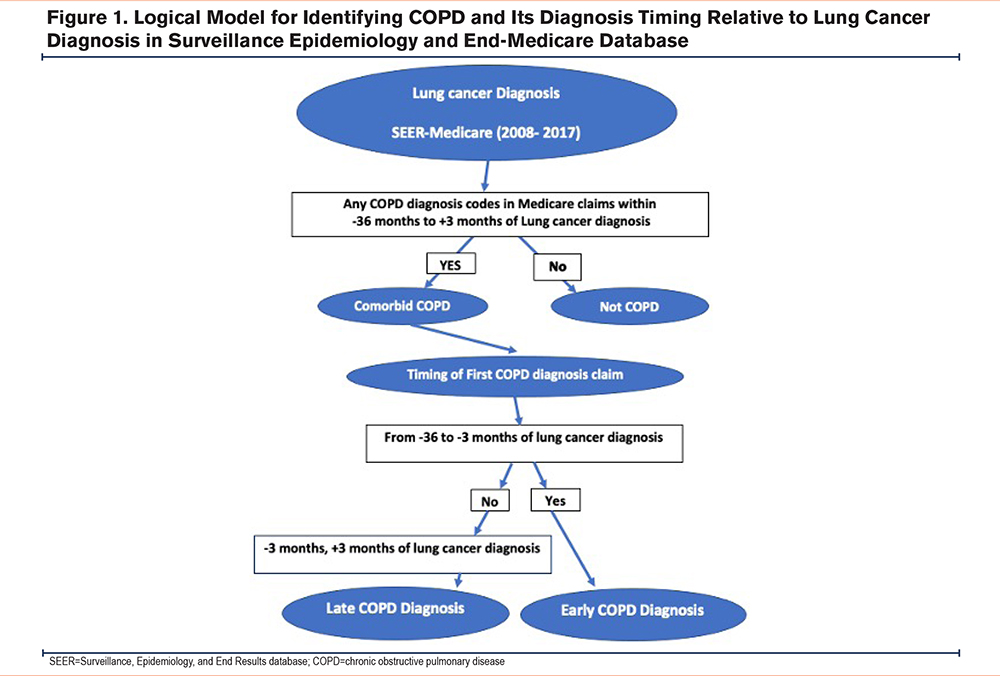
Outcome
We used the SEER summary stage to classify lung cancer stage at diagnosis into localized, regional, or distant. Further, we used the Tumor, Node, Metastasis Staging System (8th edition) from the American Joint Committee on Cancer to classify the SEER stage into early (localized SEER stage) versus late stage (regional and distant SEER stage).26 The outcome of interest was early-stage versus late-stage lung cancer at diagnosis
Covariates
We examined sociodemographic characteristics from the SEER registry including:
- Age at lung cancer diagnosis;
- Sex (female/male);
- Race and ethnicity categorized as NHW, NHB, Hispanic, American Indian/Alaskan Native (AIAN), Native Hawaiian or Pacific Islander (NHPI), Asian American, mixed race and other race;
- Marital status (married/partnered versus not);
- Census-tract indicators of SES (Yost U.S.-based quintile) and poverty27;
- County rurality (metro, urban, rural);
- SEER registry region (East, South, Midwest, West);
- Year of lung cancer diagnosis (2008–2017).
Tumor characteristics included histology: non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), and other invasive lung cancer, and tumor stage using SEER summary stage.
Clinical characteristics from Medicare claims included health care utilization including frequency of inpatient stays, outpatient, and emergency department visits in the year prior to the index date. We used a case definition algorithm to identify the occurrence of an acute exacerbation of COPD (AECOPD) in the year before the index date as per the following: one or more occurrences of diagnosis codes (ICD-9 518.81, 518.82, 518.84, 786.09,799.1, 491.21, 491.22 or ICD-10 J44.1). We estimated comorbidity burden using the Klabunde modified Charlson Comorbidity Score Index (CCI)28 using claims in the 12 months before the index date, classifying patients into 4 groups (0, 1, 2, 3+) based on the comorbidity score for each patient. Our comorbidity score excluded COPD so we could compare patients with and without COPD.
Statistical Analysis.
We calculated descriptive statistics of sociodemographic, clinical, and tumor characteristics comparing lung cancer patients with and without COPD and based on timing of a COPD diagnosis (preexisting versus concurrent) relative to the index date. We used generalized linear models with Poisson distribution and identity link (to estimate prevalence difference [PD]) and log link (to estimate prevalence ratio [PR]) and corresponding 95% confidence intervals (CIs) for the association between a preexisting (versus concurrent) COPD diagnosis with early (versus late) lung cancer stage at diagnosis among patients with COPD only. We fit 2 models: age adjusted and a fully adjusted model for age, sex, area-level SES, year of diagnosis, SEER region, CCI, and frequency of health care utilization. We did not adjust for race in our regression models to avoid ignoring its discriminatory effect on the association,29 but instead we reported PD and PR across all measured race and ethnicity groups. We also conducted subgroup analysis to examine possible modification of the association by sex, race and ethnicity, and SES using the same method reported above for the main association PD and PR. We considered p-value <0.3 to be sufficient to reject the null hypothesis for the subgroup analyses.
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc, Cary, North Carolina). This study was reviewed and approved as exempt by the University of North Carolina at Chapel Hill Institutional Review Board (#22–2998). We followed the “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines for reporting observational studies.30
Results
Our final study sample included 159,542 patients with lung cancer diagnosed between 2008 and 2017 (Figure 2). The mean age (SD) was 77.6 (6.6) years, the population has slightly more females (51.5%) than males, 85% of patients were NHWs, 6.7% were NHBs, 3.8% were Hispanic, 3.8% were Asian, 0.3% were AIAN and 0.2% were of NHPI race and ethnicity. Reflecting the SEER regions, 83.4% of patients were residents of metropolitan areas, 22.4% lived in areas of highest poverty, and 16.5% lived in the lowest SES neighborhood quantile. Lung cancer was diagnosed at an early stage for 21.5% of patients, 79.6% were diagnosed with NSCLC, while 11.1% had SCLC (Table 1).
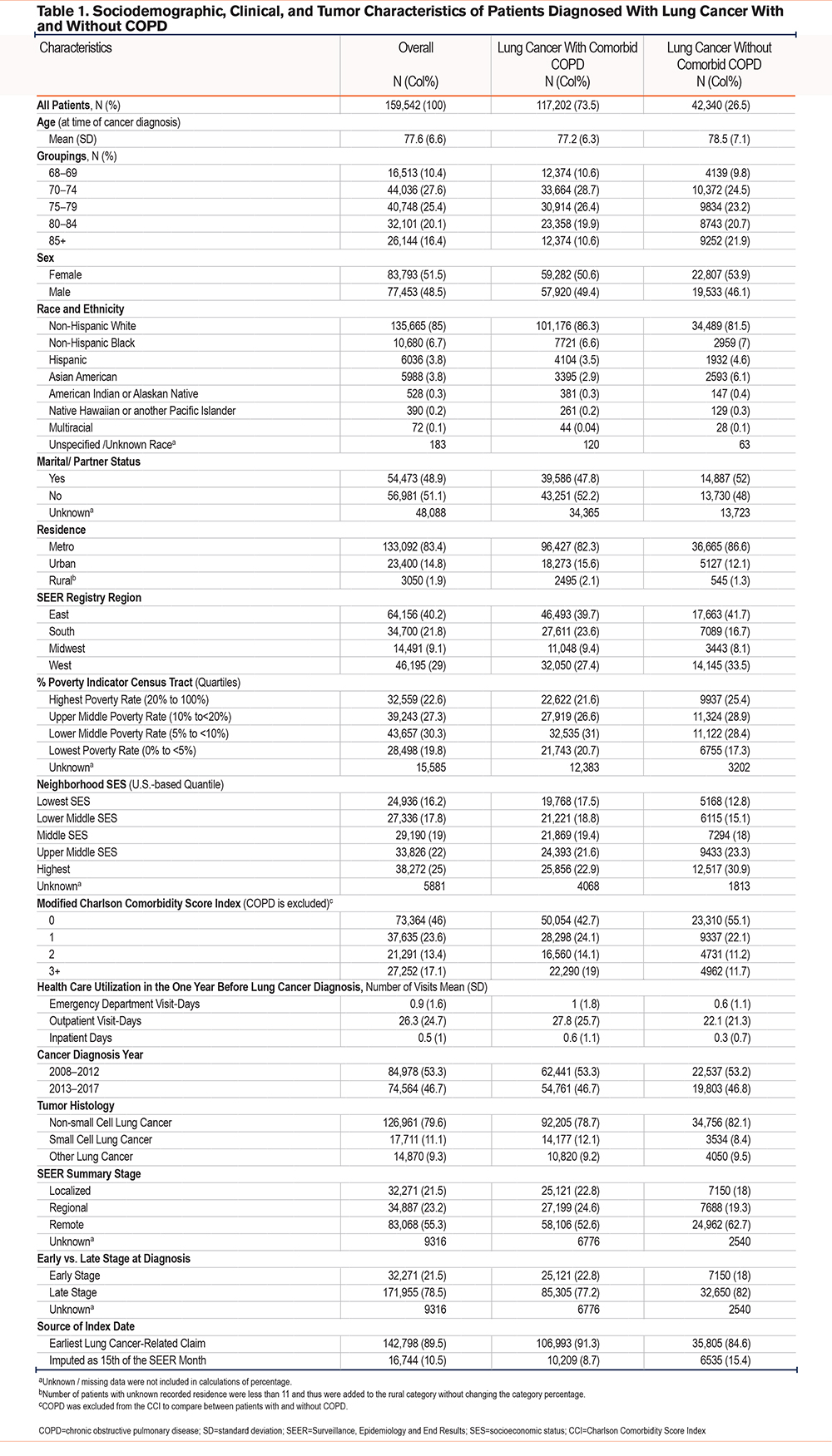
A total of 117,202 (73.5%) of our study patients had evidence of a COPD diagnosis in the 36 months prior to 3 months after their index date. Compared to patients without COPD, patients with COPD were more likely to be younger, male, more NHWs (86.3% versus 81.5%), less NHBs (6.7% versus 7%), less Hispanic (3.5% versus 4.6%), and more likely to live in poverty and in low SES neighborhoods. Clinically, they were more likely to have 3 or more comorbidities other than COPD (19% versus 11.7%), more health care utilization related to any medical condition at inpatient, outpatient, and emergency department settings, and more likely to get diagnosed with early-stage lung cancer (22.7% versus 18%) compared to patients without comorbid COPD (Table 1).
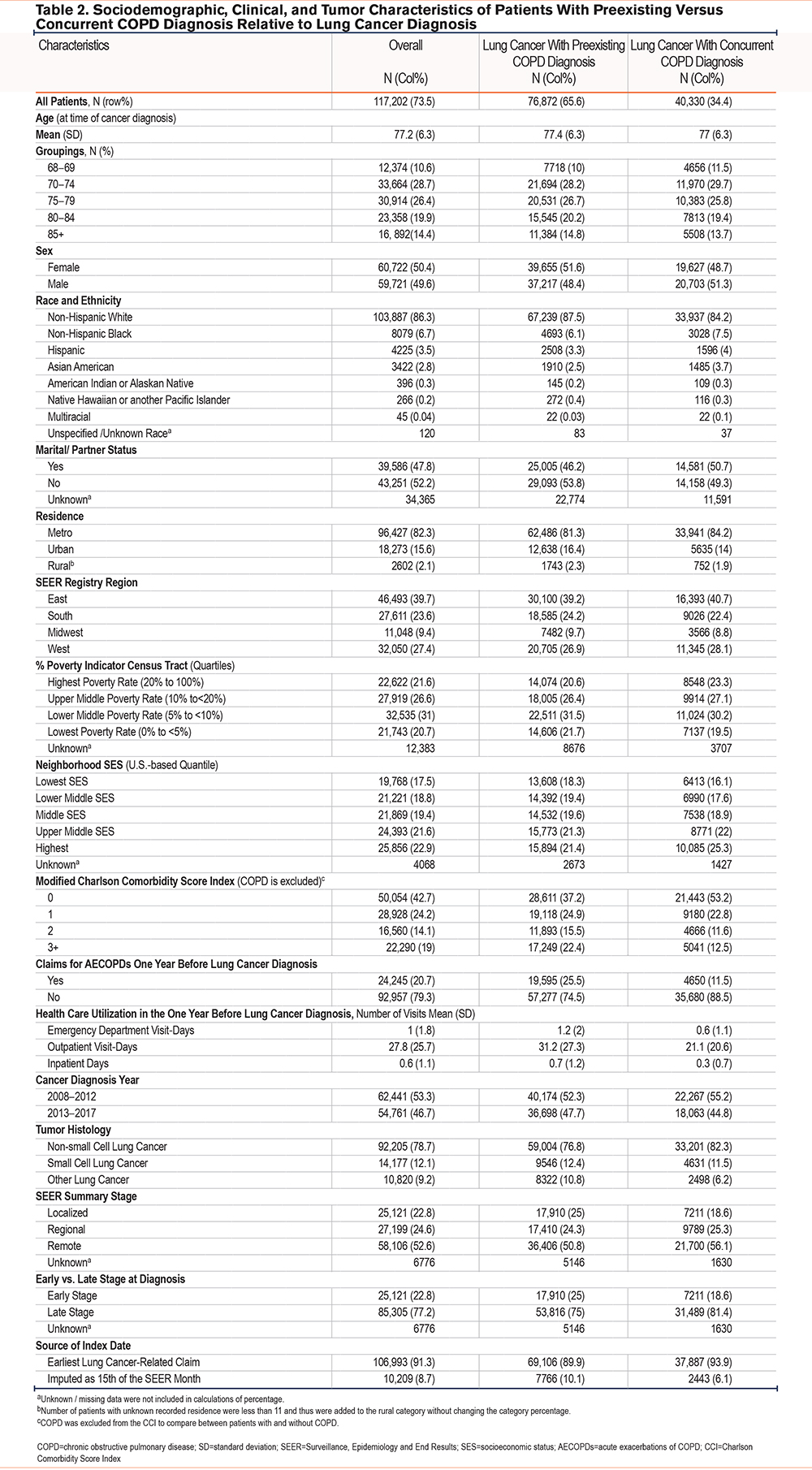
Among 117,202 patients with comorbid COPD and lung cancer, 76,872 (65.6%) had a preexisting COPD diagnosis (evidence of COPD-related health care utilization >3 months before index date), while 40,330 (34.4%) had a concurrent COPD diagnosis (evidence of COPD-related health care utilization only during the +/- 3 months from the index date). Patients with a preexisting COPD diagnosis were more likely to be older, female, NHWs, and had a lower area level SES. Clinically, they had more comorbidities other than COPD, more health care utilization related to any medical condition at inpatient, outpatient, and emergency department settings, more AECOPDs, and they were more likely to get diagnosed with early-stage lung cancer (24.9% versus 18.6%) compared to patients with a concurrent COPD diagnosis (Table 2).
Association Analysis
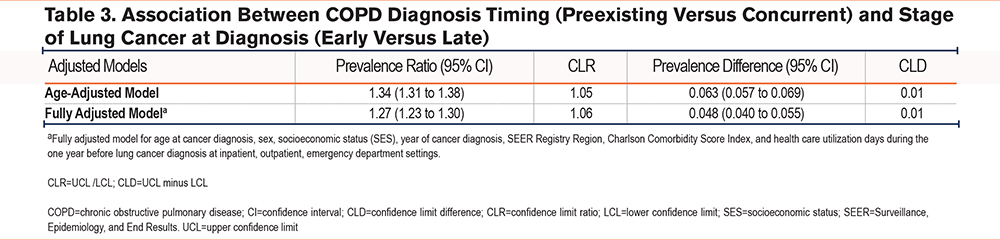
We observed a positive association between a preexisting (versus concurrent) COPD diagnosis and early-stage (versus late-stage) lung cancer at diagnosis. The age-adjusted PR (95% CI) was 1.34 (1.31 to 1.38) and PD (95% CI) was 0.063 (0.057 to 0.069). The association persisted after adjusting for age, sex, area-level SES, CCI, health care utilization during one year before lung cancer diagnosis, year of cancer diagnosis, and SEER registry region; fully adjusted PR (95%) CI was 1.27 (1.23 to 1.30) and fully adjusted PD was 0.048 (0.040 to 0.055) (Table 3).
Subgroup (Effect Measure Modification) Association Analyses
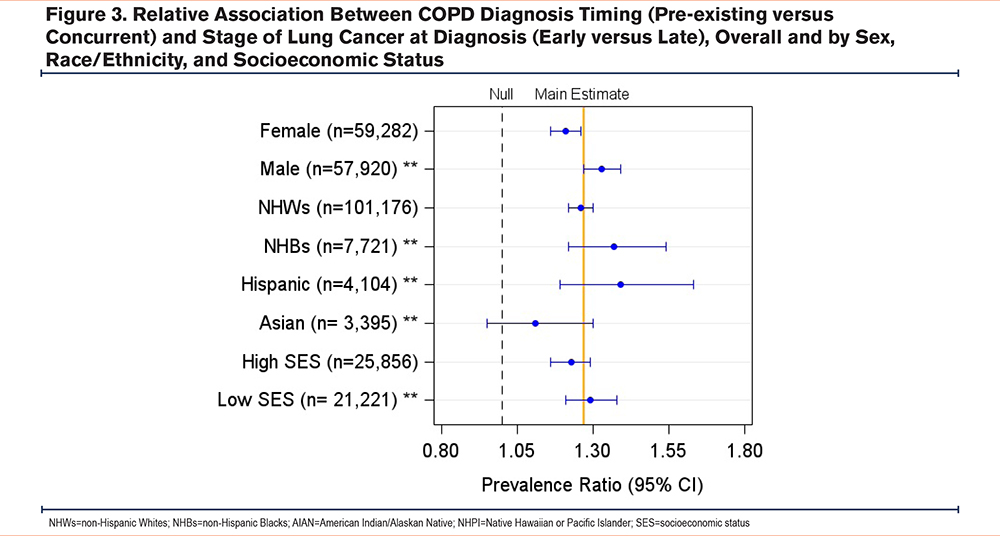
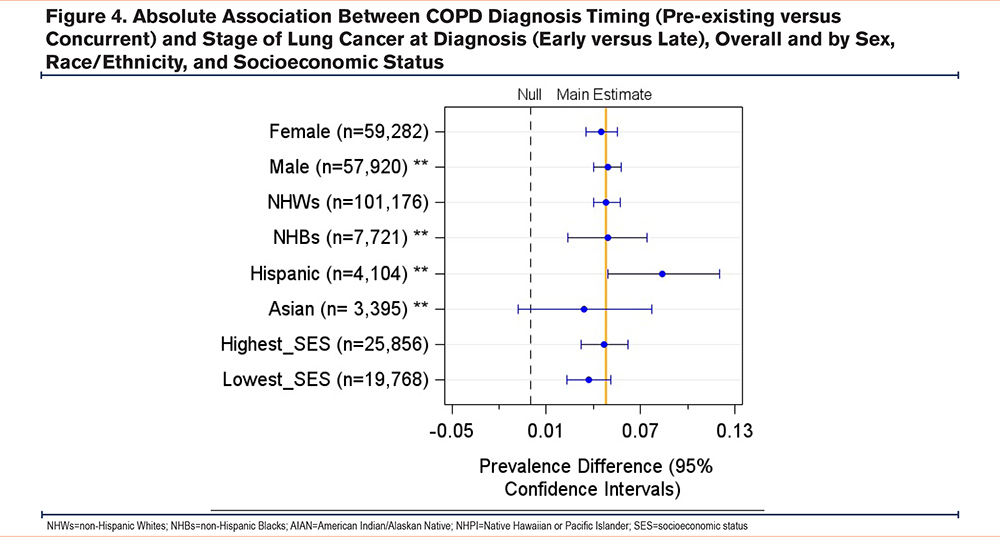
We observed some heterogeneity in the association between the timing of a COPD diagnosis and the stage of lung cancer at diagnosis across patient groups by race and sex. On the additive scale, the adjusted PD (95% CI) for NHWs was 0.048 (0.040 to 0.057), while the PD for Hispanics was 0.084 (0.049 to 0.120), and the difference in PDs (interaction contrast) between Hispanics and NHWs was 0.036 (-0.0002 to 0.072). On the multiplicative scale, the adjusted PR for NHBs was 1.37 (1.22 to 1.54), while the PR for NHWs was 1.26 (1.22 to 1.30) and the ratio of NHBs to NHWs PRs was 1.11 (0.94 to 1.30). The adjusted PR (95% CI) for males was 1.33 (1.28 to 1.39), while for females was 1.21 (1.16 to 1.26), and the ratio of male to female PRs (95% CI) was 1.09 (1.04 to 1.16). In addition, there was some heterogeneity observed across patient groups by census-tract level of SES (Yost U.S.-based quintile) (Supplemental Table 1 in the online supplement) (Figures 3, 4).
Discussion
The present study responds to several calls9,10,31,32for population-based evidence regarding the underdiagnosis of COPD among patients with lung cancer in the United States. We studied prevalence and diagnosis timing of COPD relative to the start of the lung cancer diagnostic episode, approximated by an index date, among older Medicare beneficiaries with lung cancer. We found that three-quarters of patients with lung cancer had comorbid COPD, of whom two-thirds had a COPD diagnosis at least 3 months before their lung cancer diagnosis (preexisting COPD), while the remainder had their COPD diagnosis around the time (-/+ 3 months) of their lung cancer diagnosis. Among patients with comorbid COPD and lung cancer, a preexisting COPD diagnosis was positively associated with early-stage lung cancer diagnosis, particularly among Hispanics and NHBs (compared to NHWs) and for males (compared to females).
Previous estimates25,33,34 of a comorbid COPD diagnosis among patients with lung cancer varies widely between 20% to 90%.This might be due to inconsistent diagnostic indicators of COPD used across practices where COPD is often self-reported or electronic health records documented without evidence of obstructive pulmonary function testing.35 In the present study, more than one-third of patients with comorbid COPD and lung cancer did not have evidence of their COPD diagnoses until +/-3 months of a lung cancer diagnosis. There is a scarcity of data about the timing of a COPD diagnosis relative to a lung cancer diagnosis in the United States, however, a few studies from other countries reported a high rate of COPD underdiagnosis at the time of lung cancer diagnosis. A recent study of administrative data linked to a cancer registry from Canada observed that 18.5% of patients were undiagnosed with COPD until +/-3 months of a lung cancer diagnosis. They reported 30% lower odds of a late-stage diagnosis among patients with COPD, especially those with a preexisting COPD diagnosis.25 In a study from Spain, 60% of patients had their COPD diagnosed less than 6 months before their lung cancer diagnosis.34 In another study from Spain, 71.5% of patients had their COPD diagnosed around the time of their lung cancer diagnosis.36 A study from China reported a very high rate (93%) of COPD underdiagnosis at the time of lung cancer diagnosis.37 Some of these studies reported a higher proportion of early-stage lung cancer among patients with a preexisting COPD diagnosis, but only one25 of them quantified the association between timing of a COPD diagnosis and the stage of lung cancer at diagnosis.
Our adjusted models indicated a positive association between a preexisting COPD diagnosis and early-stage lung cancer at diagnosis among patients with both conditions. Patients with a preexisting COPD diagnosis had several characteristics that may have increased their chance of health care encounters, and accordingly early-stage lung cancer, such as being female, NHW, suffering from more comorbidities and AECOPDs, and having higher health care utilization at inpatient, outpatient, and emergency department settings before a lung cancer diagnosis compared to patients with a concurrent COPD diagnosis. These characteristics are frequently associated with a higher incidence of early-stage lung cancer through symptomatic or incidental diagnosis, or through participation in a lung cancer screening.38
Our subgroup analyses revealed that males, Hispanics, and NHBs were more likely to have an early-stage lung cancer diagnosis when they had preexisting COPD compared to women and NHWs. These findings are critical because racial minorities have higher incidence, late-stage at diagnosis, and mortality from lung cancer, compared to NHWs in the United States.11,39-41 Lung cancer is the leading cause of death among NHBs and Hispanic men and the second leading cause of death among Hispanic women.42 Moreover, several studies have reported substantial racial disparities with COPD diagnoses among Black populations in the United States. In a cross-sectional analysis of the COPD Genetic Epidemiology (COPDGene®) study, NHBs had a higher chance of undiagnosed COPD regardless of the severity of airflow limitation compared to NHWs.16 For sex differences, several studies reported that men are more likely to have a concurrent COPD diagnosis16 and late-stage lung cancer diagnosis compared to women.43
Several factors might explain underdiagnosis of COPD such as nonspecific COPD symptoms that are often unrecognized or attributed to advancing age or smoking by the patient44 or overlooked by the health care provider who is focusing on management of other health conditions commonly cooccurring in elderly populations.45 Further, underutilization of spirometry to confirm COPD diagnoses in primary care settings might affect the consistency of COPD diagnosis and management.35 In the context of lung cancer, the underdiagnosis of COPD might have critical implications such as decreased motivation to quit smoking, progression of the disease, decreased eligibility for lung cancer surgical treatment, and increased health care utilization.46 However, previous studies34,47reported that the majority of patients with comorbid COPD have mild to moderate severity of airflow limitation which make them eligible for surgical treatment when their lung cancer is diagnosed at an early stage.
Strengths and Limitations
We used the large population-based data of SEER-Medicare which represent ~ 40% of the U.S. population 65 years and older and include populations of diverse racial and SES backgrounds. Our findings of a positive association between COPD diagnosis timing and stage of lung cancer are novel and should inform ongoing research for early detection of lung cancer. However, our study is not without limitations. First, our use of medical claims to identify COPD diagnoses could be prone to misclassification. To minimize misclassification bias, we used a diagnosis algorithm with a sensitivity of 85% and a specificity of 78.4% used across multiple settings.48 Second, it is impossible to know for certain that the earliest ICD code observed during the study period reflects incident COPD diagnosis. Therefore, we limited our study population to those with at least 3 years of continuous enrollment to capture all possible health care encounters before the lung cancer diagnosis. Our approach aligns and improves upon SEER-Medicare recommendations of requiring at least 2 years of claims data to conclude evidence of a first diagnosis of chronic conditions such as COPD.49 Third, we lacked data about COPD severity and smoking which might better explain the association between the timing of a COPD diagnosis and the stage of lung cancer at diagnosis. However, we adjusted for other important confounders that might contribute to this association such as health care utilization during the year before the lung cancer diagnosis and comorbidities. In addition, we conducted a simple quantitative bias analysis for unmeasured smoking and COPD severity, and it did not fully account for the measured association (Online Supplement).
Conclusions
Approximately 7 out of 10 patients with lung cancer in the United States had comorbid COPD; of whom one third did not receive their COPD diagnosis until around the time of their lung cancer diagnosis. A timely COPD diagnosis is associated with early-stage lung cancer at diagnosis, especially among high-risk populations such as racial minorities in the United States. Our findings add to the growing body of evidence about the importance of a timely COPD diagnosis to achieve an earlier stage of lung cancer diagnosis and better patient outcomes.
Acknowledgements
EMM, CAT, JLL, MBD, and CP contributed to study conception, design, and interpretation. SPH contributed to data acquisition and analysis. EMM, CAT, and CP contributed to the analysis. EMM drafted the report, and all authors revised it critically. SPH is responsible for data integrity of this study.
California Cancer Registry Acknowledgment: The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors.
Declaration of Interests
EMM receives salary and research support from the National Institute of Environmental Health Sciences: 2T32ES007018. CAT receives salary support from the University of North Carolina at Chapel Hill and research support from the National Cancer Institute, the National Library of Medicine, the Centers for Disease Control and Prevention, Academy Health, and the Gordon and Betty Moore Foundation. She has no other financial interests to disclose. JLL receives funding from an F-Hoffman La-Roche grant. Her spouse was formerly employed by and owned stock in GSK. MBD, CP, and SHP have no relevant conflicts of interest related to this manuscript.