Running Head: COPD and Osteoporosis
Funding Support: No funding.
Date of Acceptance: March 29, 2024 | Publication Online Date: April 16, 2024
Abbreviations: Cd2+=cadmium; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; GWASs=genome-wide association studies; ICS=inhaled corticosteroid; IVW=inverse variance-weighted; MR=Mendelian randomization; OR=odds ratio; SE=standard error; SNPs=single nucleotide polymorphisms
Citation: Dou Z, Chen X, Chen J, Yang H, Chen J. Chronic obstructive pulmonary disease and osteoporosis: a two-sample Mendelian randomization analysis. Chronic Obstr Pulm Dis. 2024; 11(4): 416-426. doi: http://doi.org/10.15326/jcopdf.2024.0501
Online Supplemental Material: Read Online Supplemental Material (128KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by nonreversible lung diseases that progress to emphysema or chronic bronchitis.1 Unfortunately, these conditions cannot be completely reversed and can result in significant illness and mortality. In the United States, COPD is a prominent cause of death, ranking sixth among all causes.2 In 2019, the global prevalence of COPD was estimated using the Global initiative for chronic Obstructive Lung Disease (GOLD)3 case definition among individuals aged 30–79 years, resulting in a prevalence of 10.300% (95% confidence interval [CI] 8.200–12.800).4 This led to the reporting of 212.3 million prevalent cases of COPD worldwide, with 3.3 million deaths and 74.4 million disability-adjusted life years attributed to the disease5 in 2019.
Despite a reduction in its burden (from 1990 to 2019, in almost all sociodemographic regions, the age-standardized disability-adjusted life years and death rates of COPD due to smoking and secondhand smoke, occupational particles, low temperature, and household air pollution, were presented consistently as declining trends), COPD continues to pose a significant public health challenge.6 Patients with COPD have a higher likelihood of being diagnosed with cardiovascular disease compared to those without COPD. They are at a 2 to 5 times increased risk of conditions such as cardiac dysrhythmia, heart failure, pulmonary circulation diseases, and ischemic heart disease. Patients with COPD are more prone to reporting hypertension (odds ratio [OR] 1.330; p-value<0.001) and diabetes (OR 1.360; p-value<0.001) than individuals without the condition. These findings underscore the need for effective management and prevention of COPD, as well as appropriate monitoring and treatment of associated comorbidities.7
The impact of osteoporosis on global public health is significant due to fractures leading to morbidity and mortality. While postmenopausal osteoporosis is prevalent, secondary osteoporosis affects up to 30% of postmenopausal women, > 50% of premenopausal women, and between 50% and 80% of men.8 It is crucial to exclude secondary causes, as treating the underlying condition is often the first step in the treatment of these patients.8 There have been multiple studies indicating that COPD has systemic effects that are correlated with an elevated risk of osteoporosis.9-12 However, it should be noted that all of the studies conducted thus far have been observational, and there is a lack of studies providing genetic evidence to support the association between COPD and osteoporosis.
COPD and osteoporosis are complex diseases with multiple factors contributing to their development. There may be some common mechanisms or pathways between these 2 diseases, making it difficult to determine the nature of their relationship. The association between COPD and osteoporosis observed in studies may be causal, but could also be influenced by confounding bias, meaning that there may be other factors that influence the results of studies. Therefore, while observational studies provide important insights, they cannot establish causality alone, and more research is needed to determine if there truly is a causal relationship between these 2 conditions.
Mendelian randomization (MR) is a statistical method that utilizes genetic variants as instrumental variables to investigate the causal associations between an outcome of interest and a modifiable risk factor. MR can guard against some of the critical limitations that arise in conventional observational studies, particularly confounding and reverse causation biases.13 By using genetic variants to randomly allocate individuals to different levels of the modifiable risk factor, MR can provide a more robust and less biased estimate of the causal effect of the risk factor on the outcome of interest.
Compared to conventional observational studies, MR offers a key advantage by enabling researchers to utilize publicly available data from large-scale genome-wide association studies (GWASs) for both the exposure and the outcome.14 We performed an MR analysis to probe the causal link between COPD and osteoporosis.
Methods
Study Design and Data Sources
An MR analysis model was used to evaluate the causal effect of COPD on osteoporosis as illustrated in Figure 1. Instrumental variables for COPD were derived from the largest dataset of the GWAS database, with the largest European sample size to date involving 55,907 clinically diagnosed cases and 297,408 controls, and 12,321,875 single nucleotide polymorphisms (SNPs) sequencing data (ID: ieu-b-106).15
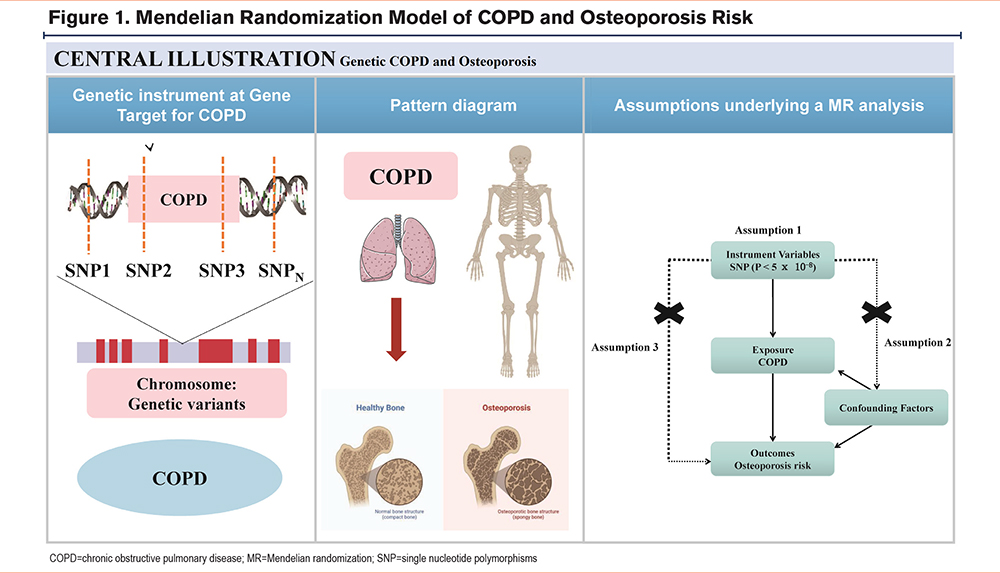
The outcome being analyzed was osteoporosis, and the study used summarized statistics data from the largest GWAS, which included 5266 clinically diagnosed cases, and 331,893 controls, and 10,894,596 SNP sequencing data (ID: ukb-a-87).15 As this study only utilized the published data from these GWAS meta-analyses, there was no additional need for ethical approval of this study.
Single Nucleotide Polymorphisms Selection
To identify the SNPs associated with COPD, we utilized a recently published GWAS database. The initial screening criteria for SNPs were: (1) autosomal biallelic SNPs with a p-value less than 5 × 10−8, and (2) minor frequency greater than >1%. Also, by clumping these 2672 SNPs together which had linkage disequilibrium, the independence of the selected genetic variants could be identified (R2 < 0.001 at a 10,000kb window). Finally, we identified 108 independent SNPs associated with COPD.
The data on osteoporosis (ID: ukb-a-87) was obtained from a recently conducted genome-wide association study involving 337,159 individuals of European descent, with 5266 cases and 331,893 controls. To adhere to the MR hypothesis, we conducted a comprehensive literature review of osteoporosis risk factors from previous studies.16-18 We then utilized the PhenoScanner V2 web to identify 108 SNPs that were significantly relevant.19 The Supplemental Table 1 in the online supplement contains comprehensive information about all the SNPs utilized in our study.
Statistical Analysis
To assess the causal impact of exposure (e.g., genetic variation) on outcomes (e.g., disease) while controlling for potential confounding factors, we used 7 MR analysis methods (simple median, weighted median, penalized weighted median, inverse-variance weighting [fixed and random effects], and MR Egger) in this study. The combination of these methods can help provide more robust and accurate estimates of causal effects in MR analyses.
To analyze the results, we followed a robust inverse-variance weighted (IVW) method that provides a causal estimate even in the presence of heterogeneity. IVW method was a valid analysis mainly performed in accordance with the basic premise that all genetic variants have a strong ability to detect causality. The weighted median estimator was used to produce robust causal estimates; the weighted median method can provide accurate calculation results even if over 50% of instrumental variables were invalid. The MR-Egger method mainly considered the horizontal pleiotropy of instrumental variables, which included an intercept term in the regression model to quantify the directional pleiotropy. An intercept term that was considerably different from zero in statistics revealed the presence of pleiotropy and a breach of the basic MR assumption. However, because the MR assumptions were required for all instrumental variables in the IVW method, additional sensitivity analyses using the weighted median estimator and MR-Egger were carried out. Additionally, to evaluate the potential pleiotropy and heterogeneity of individual SNPs, we applied IVW approaches incorporating MR Egger intercept and Cochran’s Q statistics. In the absence of significant deviation from 0 (p>0.05) for the intercept, the absence of pleiotropic effects was considered. Heterogeneity was determined using the value of Cochran's Q, with the primary outcome being the application of the IVW method with a multiplicative random-effects model when the p-value was < 0.05, and the use of the IVW method with a fixed-effects model as the primary outcome when the p-value was ≥ 0.05.
In this study, we performed MR-Egger regression, which has the ability to detect and adjust pleiotropy, obtain a causal effect assessment, and determine whether directional horizontal pleiotropy is responsible for the results. Therefore, IVW and MR-Egger regression were the most important analysis methods for the MR study. Consistent with previous research, 3 conditions must be met for a causal relationship to be deemed significant: (1) a p-value of less than 0.05 in the IVW method, (2) no significant difference in the direction of estimates among the weighted median methods, IVW, MR-Egger, and (3) a p-value greater than 0.05 in the MR-Egger intercept test. We performed a leave-one-out analysis to evaluate the reliability of our MR analysis outcomes and identify any SNP that may have an undue influence on the results.
The software utilized for statistical analysis was the “TwoSampleMR” package of R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was determined with a 2-tailed p value <0.05.
Results
COPD on Osteoporosis
Supplemental Table 1 in the online supplement provides a comprehensive description of all the genetic datasets utilized in this study. To examine the outcomes, we conducted 7 MR analysis methods, including inverse-variance weighted (fixed-effect and random-effect), simple median, weighted median, MR Egger, MR Egger (bootstrap), and penalized weighted median. The final results were analyzed using IVW MR methods, as shown in Figure 2. Additionally, to validate the IVW analysis results' reliability, we utilized the remaining MR analysis methods (simple median, weighted median, MR Egger, MR Egger [bootstrap], and penalized weighted median) as supplementary analyses, as depicted in Figure 3.
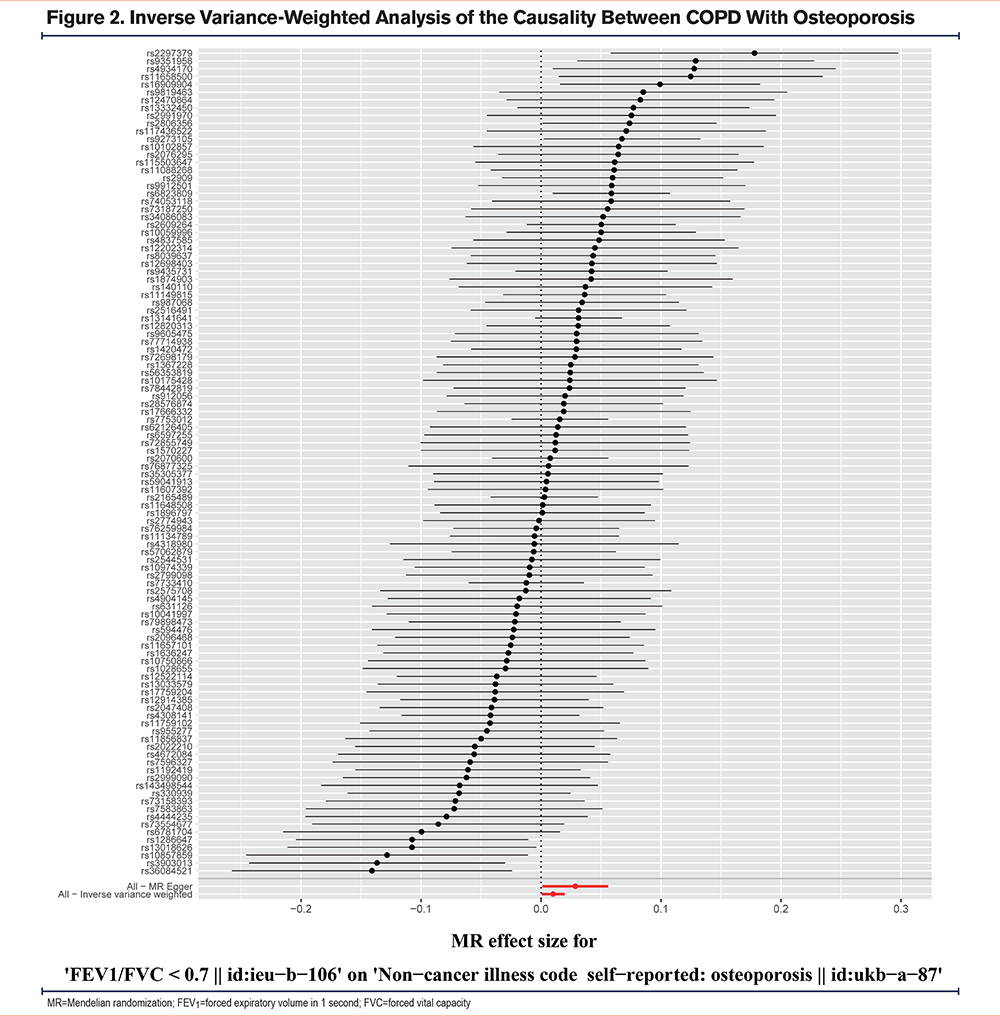
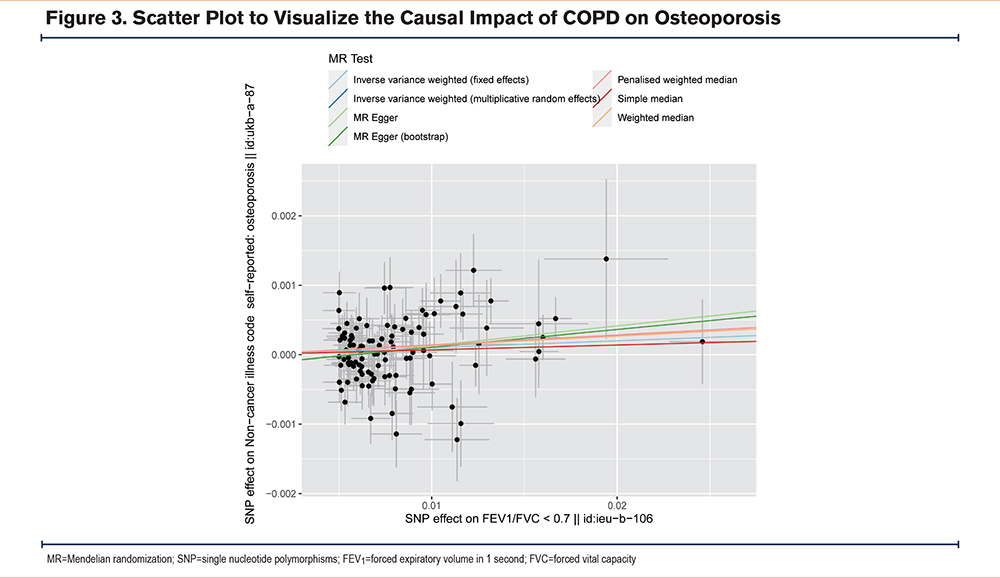
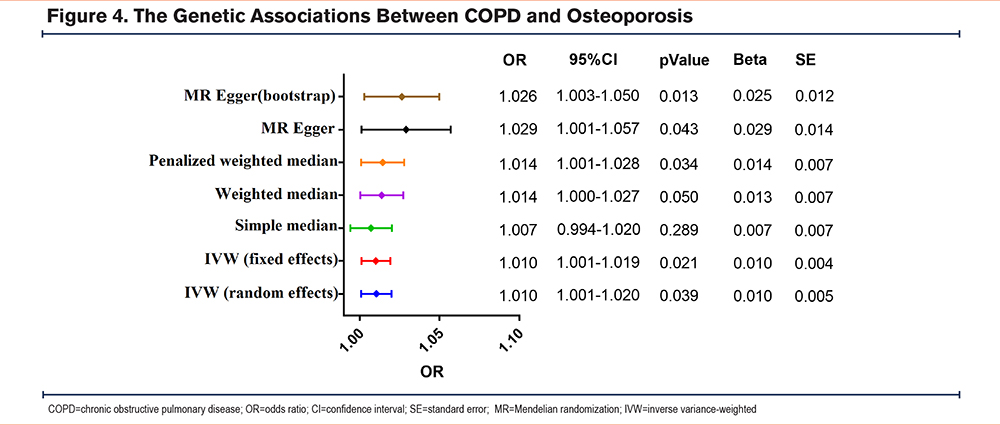
Various statistical methods, including Cochran's Q statistics, MR Egger intercept, and IVW methods, were employed to assess the heterogeneity and pleiotropy of the SNP. The MR analysis was conducted using the fixed-effect IVW model and random-effect IVW model, which revealed a significant association between COPD and osteoporosis. Specifically, using 108 SNPs as instrumental variables with a significant difference, the ORs for this association were 1.010 (95% CI, 1.001–1.019, p-value, 0.021) and 1.010 (95% CI, 1.001–1.020, p-value, 0.039), respectively. Furthermore, our study analyzed the results using various methods, including simple median (OR, 1.007; 95% CI, 0.994–1.020, p-value, 0.289), weighted median (OR, 1.014; 95% CI, 1.000–1.027, p-value, 0.050), penalized weighted median (OR, 1.014; 95% CI, 1.001–1.028, p-value, 0.034), MR-Egger method (OR, 1.029; 95% CI, 1.001–1.057, p-value, 0.043), and MR Egger (bootstrap) method (OR, 1.026; 95% CI, 1.003–1.050, p-value, 0.013) (Figure 4). The findings from these analyses showed similar results, with the ORs ranging from 1.007 to 1.029 (Supplemental Table 1 in the online supplement).
The IVW analysis (Q=131.374, p-value=0.061) and MR-Egger analysis (Q=128.895, p-value=0.069) provided no evidence of heterogeneity. The MR-Egger regression analysis did not provide evidence of any directional pleiotropic effect across genetic variants, as evidenced by the intercept (-0.0002; P=0.160). In Figure 5, the results of the leave-one-out sensitivity analysis suggested that no individual SNP substantially drove the association between COPD and osteoporosis. Furthermore, as asymmetry in the funnel plot can bias MR methods by indicating directional horizontal pleiotropy, we examined the funnel plot for evidence of asymmetry but found no such evidence in our study (Figure 6). Overall, our findings suggest a robust and reliable association between COPD and osteoporosis.
Discussion
This study represents the first attempt to investigate the causal relationship between genetically predicted COPD and osteoporosis using an MR approach. The MR analysis conducted in this study provides evidence of a causal association between genetically determined COPD and increased risk of osteoporosis. Specifically, the findings suggest that individuals with a higher genetic predisposition for COPD are at an elevated risk of developing osteoporosis; a population with COPD has a 1%–3% increased risk of osteoporosis compared to a population without COPD. The study’s findings could have significant implications for the prevention and management of osteoporosis in patients with COPD.
One of the extrapulmonary effects of COPD is osteoporosis. Hip fracture due to osteoporosis is associated with diminished quality of life and is the most important risk factor for mortality in elderly persons.20 In the last 10 years, numerous observational investigations have been conducted to offer convincing evidence for the link between COPD and osteoporosis. According to Graumam et al patients with COPD had a significantly higher prevalence of osteoporosis compared to healthy controls (40.4% versus 13%; p=0.001). Additionally, the study found that vertebral fractures were observed in a sizable proportion of men (24.4%) and women (22%) with COPD.21 These results supported the previous findings of the Silva et al study.22 In a systematic review and meta-analysis of 58 studies related to osteoporosis, Chen et al found that COPD patients had a global prevalence of osteoporosis of 38% (95% CI, 34–43). Moreover, the presence of COPD was found to be associated with an increased likelihood of having osteoporosis (OR, 2.83).23 These results provide compelling evidence to support the relationship between COPD and osteoporosis.
It is possible that there is not one exact pathway in which COPD leads to osteoporosis, but that multiple pathways are involved. Earlier studies have indicated that COPD is a condition of systemic inflammation, which is linked to a higher prevalence of comorbid cardiovascular diseases. These conditions may include, but are not limited to, heart failure, hypertension, ischemic heart disease, diabetes, cardiac dysrhythmia, and pulmonary hypertension.7,24 However, cardiovascular disease is known to cause osteoporosis,25 and many drugs used to treat cardiovascular disease can also cause osteoporosis, such as anticoagulant agents medications (warfarin, heparin),26,27 as well as antihypertensive medications.28 Therefore, whether osteoporosis is caused by COPD itself or by its cardiovascular complications remains a major challenge for observational studies to further investigate. In addition, inhaled corticosteroids (ICSs) are widely used in COPD patients and it is now clear that corticosteroid medication is one of the most important risk factors for osteoporosis.29 In the ARCTIC study, a relationship between dose and effect was observed. High-dose ICS use was significantly associated with any osteoporosis-related event (risk ratio 1.52; 95% CI 1.24–1.62), while low-dose ICS use had a corresponding estimate of 1.27 (95% CI 1.13–1.56) when compared to COPD patients not using ICSs.30 These findings indicate that patients with COPD have an elevated risk of bone fractures and osteoporosis, and the use of high-dose ICSs may further increase this risk. Therefore, health care providers should be aware of these risks and carefully consider the use of ICSs when treating COPD patients. This study confirms the important role of COPD in causing osteoporosis, and also points to the influence of ICSs on osteoporosis. These results are supported by the Adas-Okuma et al study that showed COPD as an independent risk factor for osteoporosis and fractures.31 However, the above studies were all observational studies, and one exposure factor (for example, COPD) frequently is affected by compounding factors (cardiovascular comorbidities, ICSs), likely creating result bias. Compounding factors adjustment is indispensable for investigating the causal relationships.32 Unfortunately, it is not easy to eliminate compounding interference.
MR is a rapidly growing field of genetic epidemiological methodology that utilizes genetic data to establish causality between exposure and outcome.33 Since genotype is an innate characteristic that precedes the outcome and is not influenced by other confounding factors such as acquired environmental factors, it serves as a powerful tool to determine causality.34 Unlike traditional epidemiological methods, this approach reveals the direction of the exposure-outcome relationship, providing insights into the causal relationship between the 2, rather than just their association. Our study identified a causal relationship between genetically predicted COPD and an elevated risk of osteoporosis. Based on our results and previous reports, we suggest that the causal relationship between genetically predicted COPD and osteoporosis may be mainly related to the abnormal bone metabolism induced by hypoxia and low exercise intensity. In a recent study, it was reported that hypoxia resulting from COPD induces the expression of divalent metal transporter-1, thereby facilitating the transport of cadmium (Cd2+) into cells. Cd2+ has been shown to be considerably harmful to bone and kidney health, and its detrimental effects may compromise bone microarchitecture, ultimately contributing to the development of osteoporosis.35 Another study also pointed out that osteoporosis is a frequently observed disorder of bone metabolism in patients with respiratory impairment. Hypoxia-induced reactive oxygen species accumulation and activation of hypoxia-inducible factor 1 alpha are known to result in weakened osteogenesis and augmented osteoclastogenesis in patients with respiratory disorders.36 Furthermore, in addition to its diverse complications, hypoxia and low physical activity caused by COPD are also important mechanisms of osteoporosis. COPD patients are often associated with low physical activity compared to non-COPD patients. Multiple studies have confirmed that low physical activity is an independent risk factor for low osteoporosis.37-39
Our study has several limitations that should be acknowledged. First, the data used in this study was obtained from a European database, which may restrict the generalizability of our findings to other ethnic groups. Second, the exposure and outcomes were based on the United Kingdom Biobank population, which may result in sample overlap. However, we were unable to quantify the extent of sample overlap in our study. These limitations highlight the need for further research to confirm our results in other populations and databases. Thirdly, this MR study cannot reveal the specific molecular mechanism of COPD leading to osteoporosis. Future studies are still needed to further confirm the current causal relationship mentioned in this study. Finally, the GWAS database is not inclusive of both primary and secondary osteoporosis, and this is because it is difficult to make a statistical investigation on the specific causes of osteoporosis in the genetic epidemiological investigation of a large sample of the population. Our results, therefore, confirm the causal effect of genetic predicted COPD on osteoporosis from a macro perspective. However, this result needs to be further confirmed in future studies for specific populations.
Conclusions
Based on our genetic analysis, our study provides evidence of a causal relationship between COPD and an elevated risk of osteoporosis. Given the higher likelihood of osteoporosis recurrence in patients with COPD, it is crucial to monitor these individuals regularly. Furthermore, additional research is warranted to identify preventative strategies for osteoporosis in COPD therapy.
Acknowledgements
Author contributions: JQC and HY were responsible for the study conception and study design. ZQD and JC were involved in data acquisition and study execution. JC analyzed the data and ZQD drafted the manuscript. JC contributed to interpretation and editing of the manuscript. XRC revised the manuscript. All authors read and approved the final manuscript.
Data availability statement: All data generated or analyzed during this study are included in this published article.
The authors would like to thank all participants for their great support for UK-Biobank. The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Declaration of Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.