Running Head: Bronchiectasis in Alpha-1 Antitrypsin Deficiency
Funding Support: This research is funded by Vertex Pharmaceuticals and the National Institute for Health and Care Research (NIHR) Midlands Patient Safety Research Collaboration (PSRC). The views expressed are those of the author(s) and not necessarily those of the NIHR, Vertex Pharmaceuticals, or the Department of Health and Social Care.
Date of Acceptance: August 11, 2024 | Publication Online Date: August 29, 2024
Abbreviations: AAT=alpha-1 antitrypsin; AATD=alpha-1 antitrypsin deficiency; BSI=bronchiectasis severity index; CI=constant interval; COPD=chronic obstructive pulmonary disease; EARCO=European Alpha-1 Research Collaboration; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; KCO=carbon monoxide transfer coefficient; mMRC=modified Medical Research Council; OR=odds ratio; pp=percent predicted; TLCO=transfer factor of carbon monoxide
Citation: De Soyza J, Ellis P, Newnham M, Rickard L, Turner AM. Bronchiectasis occurs independently of chronic obstructive pulmonary disease in alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2024; 11(5): 507-514. doi: http://doi.org/10.15326/jcopdf.2024.0526
Online Supplemental Material: Read Online Supplemental Material (616KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) refers to low circulating levels of the protease inhibitor alpha-1 antitrypsin (AAT). The disease is inherited in an autosomal codominant fashion, with both alleles contributing to overall circulating AAT levels. In ascending order of AAT levels, the most common allele combinations are PiZZ, PiSZ, PiSS, the carrier state PiMZ, and the healthy or “wild” phenotype PiMM.1,2 PiZZ AATD affects 250,000 people worldwide, and PiSZ affects 1.5 million, mainly in those with northern or southwestern European ancestry.3,4 Rarer variants exist but are difficult to study in large quantities. The link between AATD and chronic obstructive pulmonary disease (COPD) has been well-described as that of unopposed protease activity, particularly neutrophil elastase, causing destruction of the small airways and development of emphysema-predominant COPD.5
Bronchiectasis constitutes another form of structural airways disease, defined as abnormal dilatation of the bronchi, and symptomatically accompanied by purulent sputum production, shortness of breath, and recurrent exacerbations.6 It can, therefore, be difficult to differentiate from the chronic bronchitis of COPD, but advances in computed tomography and availability have highlighted a significant clinically relevant overlap between the 2 syndromes.7,8 As with bronchiectasis and usual-COPD, bronchiectasis and AATD-COPD are known to occur in the same patients. According to one study of 74 patients with the PiZZ phenotype, 94% had radiological evidence of bronchiectasis, and 27% had both clinical and radiological evidence.9 In one U.S. cohort, 2.7% of patients with bronchiectasis also had PiZZ AATD phenotype10; however, findings from other cohorts vary widely.11-14
The pathophysiology of AATD provides a theoretical explanation for this since the academic consensus is that bronchiectasis of any cause is provoked by a positive feedback system of airway inflammation, structural damage, and failure of mucociliary clearance, which predisposes to bacterial infection.15,16 Bacterial infection causes further airway inflammation, and the cycle continues. More recently this model has been described as a “vortex,” with all stages contributing to all others.16 AATD fits into this model by way of increased airway inflammation and structural damage caused by unopposed protease activity. However, some conclude that bronchiectasis in AATD is primarily due to emphysema, rather than being a direct consequence.17 This justifies the study of bronchiectasis in a subgroup of AATD without COPD, particularly since a recent analysis of the European Alpha-1 Research Collaboration (EARCO) database showed a bronchiectasis prevalence of 33% in AATD patients without emphysema who had cross-sectional chest imaging.18 This high prevalence supports a direct link between AATD and bronchiectasis independently of emphysema, though the article does not comment on whether patients with alternative bronchiectasis aetiologies were excluded, and there is inherent selection bias in limiting the group to those with available imaging.18
The question, therefore, remains as to whether AATD is an independent factor for development of bronchiectasis, and if so, what clinical implications might it have on disease progression? An answer to this question is of increasing importance given the wide pharmacological variety of emerging AATD treatments,19 along with European Respiratory Society guidance to prioritize the identification of AATD endotypes for the purposes of treatment planning.20
This study aims to establish the rates of bronchiectasis in a large database of patients with AATD, its radiological appearance and severity, whether it is associated with COPD or occurs independently, and assess whether it is associated with disease outcomes including lung function decline, exacerbation rate, and symptoms.
Methods
Patients with the most common AATD phenotypes (PiZZ, PiSZ and PiMZ phenotypes) were identified from the Birmingham Alpha-1 Antitrypsin Research Database, a prospectively collected database where details on data collection have been summarized previously.21 Briefly, patients have regular visits to a tertiary AATD center, most commonly annually, with symptoms, exacerbations, new medical diagnoses, and lung function tests reviewed at each visit. Data for the present study were extracted from this database and included age at presentation, sex, baseline smoking history (current, ex, or never), AATD phenotype, COPD diagnosis, bronchiectasis diagnosis, serum AAT level, mode modified Medical Research Council (mMRC) breathlessness score,22 forced expiratory volume in 1 second (FEV1), transfer factor of carbon monoxide (TLCO), carbon monoxide transfer coefficient (KCO), and median exacerbation yearly rate. In the Birmingham registry, COPD diagnosis is defined as postbronchodilator FEV1 to forced vital capacity (FVC) ratio <0.7 on spirometry with compatible symptoms, and bronchiectasis diagnosis is defined as a radiological diagnosis made on a computed tomography (CT) scan thorax report issued by a radiologist at either the referring or tertiary center, along with compatible symptoms. COPD diagnosis accuracy was checked by reviewing spirometry for postbronchodilator FEV1/FVC ratio <0.7. Bronchiectasis accuracy was checked by reviewing available CT scan images, looking for either bronchoarterial ratio >1, lack of airway tapering, or airway visibility within 1cm of the pleural surface, as per best practice guidelines from the British Thoracic Society.23 Percentage predicted (pp) values were calculated using the Global Lung Function Initiative method.24,25 Where possible, original case note files were reviewed to retrieve missing data. Rare phenotypes were excluded.
For those with 3 or more lung function measurements, lung function annual changes were calculated using linear regression, and expressed as FEV1pp annual change, TLCOpp annual change, and KCOpp annual change. Median exacerbation rates were calculated for each patient from the yearly exacerbation rate reported at each visit.
CT images of patients with bronchiectasis, along with a control group of patients not known to have bronchiectasis, were assessed for morphology as per Reid et al26 and for lobar distribution. This assessment was conducted by a physician, with 10% also read by a radiologist. Inter-rater reliability was assessed by the Cohen’s Kappa method. Bronchiectasis severity index (BSI) scores were calculated as described by the initial validation study.27
Statistical Analysis
R statistical programming language version 4.2.3 was used for all statistical analysis.28 Separate analyses were conducted for each phenotype: PiZZ, PiSZ, and PiMZ. Demographic data was compared according to bronchiectasis diagnosis using standard statistical tests. Multivariate binomial logistic regression modeling was used to assess whether the odds ratio (OR) for bronchiectasis diagnosis was related to COPD diagnosis and to serum AAT level. A subgroup analysis to look for associations of bronchiectasis in patients without a COPD diagnosis was performed; multivariate binomial logistic regression analysis was used again, with the same covariates. The outcomes of baseline FEV1, lung function annual change, and exacerbation rates were compared between those with bronchiectasis and those without, using linear regression models for lung function models. Model assumptions were assessed for linearity and normality of residuals. Covariates of age, sex, phenotype, smoking history, and COPD diagnosis were selected for use in all multivariate analyses in this study, due to their known effects on the outcome variables and their availability in the database. Zero-sum inflated Poisson models were used for exacerbation rates, due to the high proportion of patients with no exacerbations, resulting in a heavily positively skewed distribution with an excess of zeroes, which did not meet assumptions for a standard Poisson model. Multinominal regression models were calculated for mMRC score data. All models were conducted in PiZZ patients first and repeated in PiSZ and PiMZ patients if significant results were found.
Results
Descriptive Statistics
The inclusion/exclusion process is shown in e-Figure 1 in the online supplement. A total of 4 patients were excluded due to having other causes of bronchiectasis: 1 had allergic bronchopulmonary aspergillosis, 2 had childhood pertussis, and 1 had traction bronchiectasis secondary to interstitial lung disease. All 4 had the PiZZ genotype. Among those with ≥3 lung function measurements, median follow-up time was 6.36 years (IQR 3.93–11.23).
Baseline characteristics of the PiZZ, PiSZ, and PiMZ groups, stratified by bronchiectasis diagnosis, are shown in Table 1. A total of 967 patients had spirometry available for review of diagnostic accuracy of COPD: type I and II error rates were 0.6% and 1.7% respectively (e-Table 1 in the online supplement). A total of 290 patients had CT scans available for review of diagnostic accuracy of bronchiectasis: type I and II errors were 1.6% and 7.5% respectively (e-Table 2 in the online supplement).
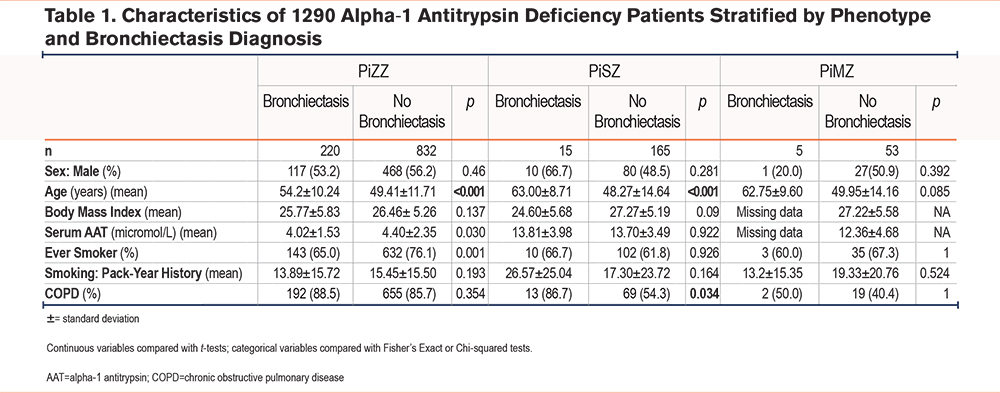
PiZZ patients with bronchiectasis were older at baseline than those without (54 versus 49 years, p<0.001), and more likely to be never smokers (35% versus 24%, p<0.001). PiSZ patients with bronchiectasis were also older on average (63 versus 48 years, p<0.001), but no differences in smoking status were seen. PiMZ numbers were small with little variance between the bronchiectasis and nonbronchiectasis groups. A total of 134 PiZZ patients did not have COPD, 25 (18.6%) of whom had bronchiectasis.
There were 174 PiZZ and 9 PiSZ patients with bronchiectasis who had CT scans available for analysis. Scans from 81 PiZZ patients and 12 PiSZ patients without bronchiectasis were also analyzed. Substantial inter-rater reliability was demonstrated by mean Cohen’s Kappa 0.67. Of the patients without a prior diagnosis of bronchiectasis, 24.7% of the PiZZ patients and 8.3% of the PiSZ patients had CT evidence of the disease. Bronchiectasis was mostly mild in both groups, with low rates of varicose and cystic bronchiectasis in the PiZZ patients (25.3% and 7.6% respectively), and exclusively cylindrical bronchiectasis in the PiSZ patients. PiZZ patients had significantly higher numbers of affected lobes (p=0.009). Morphology and lobar distribution are shown in e-Tables 3 and 4 in the online supplement.
BSI scores were calculated for those with the relevant information available from the clinical record (n=286). BSI scores were mostly mild, with 52.5% of PiZZ and 71.4% of PiSZ patients having mild disease, and only 12.5% of PiZZ and 9.5% of PiSZ having severe disease (e-Table 5 in the online supplement).
Regression Analyses
The clinical diagnosis of bronchiectasis was unrelated to a concurrent diagnosis of COPD on multivariate binomial logistic regression modeling in all phenotypes (Table 2, e-Figures 2 and 3 in the online supplement), instead having a small but significant association with older age in both phenotypes (OR 1.03, p=0.001), and smoking status in PiZZ patients, being more likely in those who have never smoked (OR 0.59, p=0.031). There was also an inverse relationship between serum AAT and bronchiectasis diagnosis in PiZZ patients (OR 0.88, p=0.018).
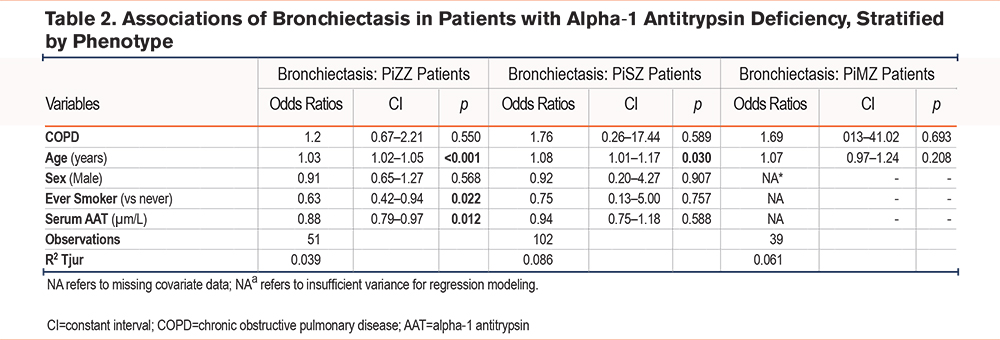
Baseline FEV1, as well as FEV1, KCO, and TLCO pp/year decline, did not differ significantly between patients with and without bronchiectasis in multivariate linear regression analysis (e-Table 6 in the online supplement). The exacerbation rate was also unrelated to bronchiectasis diagnosis (e-Table 6 in the online supplement).
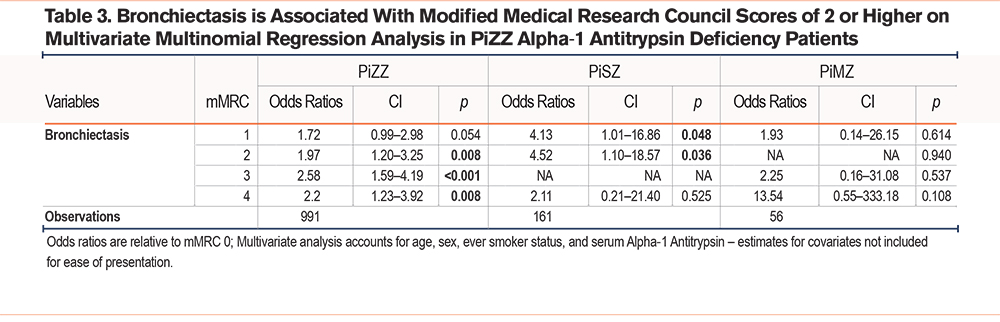
Bronchiectasis was associated with higher mMRC scores in PiZZ patients on multivariate multinomial logistic regression modelling (Table 3), however this pattern did not persist in PiSZ and PiMZ patients.
Discussion
Prevalence of bronchiectasis in our PiZZ cohort, at 20.9%, is lower than in a recently published summary of the EARCO database,18 which found a prevalence of 36.1%. However, that study used a radiological definition of bronchiectasis, whereas the present work has used a clinical code, which will likely underestimate the prevalence of radiological bronchiectasis, as demonstrated in our incidental finding of bronchiectasis in 7.5% of those not previously known to have it. Notably, radiological bronchiectasis of any cause does not necessarily bear clinical relevance unless subcategorized further,27which is why we chose to look at clinical diagnosis as our primary means of assessment. In a secondary analysis, we also subcategorized radiological bronchiectasis in order to obtain BSI, which demonstrated largely mild disease. This contrasts with the only reported use of the BSI in usual COPD patients, which found the “severe” category to be the most numerous29; however, this study used primary care patients, of whom only the most symptomatically severe patients will have CT scans. Ours is a tertiary cohort of a much rarer disease, with a proportionally lower threshold for CT scanning and consequently more incidental findings of mild bronchiectasis. This is likely to explain the lack of associations between bronchiectasis and lung function decline or exacerbation rate in our cohort. Nonetheless, a significant minority of PiZZ patients had severe and extensive disease, despite no other known cause for their bronchiectasis.
In 2012, 211,000 of an estimated 51.7 million adults in the United Kingdom had bronchiectasis,30,31 a prevalence of 0.4%. This would confirm that prevalence of bronchiectasis in our cohort is much higher than the general population, but this figure alone does not necessarily support an independent association of bronchiectasis with AATD: it is consistent with some estimates of the prevalence of bronchiectasis in usual COPD,32 though such estimates vary widely,33-34 from 0.03% to 57.6%. We, therefore, studied bronchiectasis in those without a diagnosis of COPD; the prevalence of bronchiectasis of 18.6% in this group is also much higher than in the general population, even after removing those with other clear causes, which supports an independent association of AATD with bronchiectasis. Further analysis was, therefore, warranted.
In our analysis of the associations of a bronchiectasis diagnosis, we found that bronchiectasis occurs independently of COPD in our PiZZ patients, instead being related to age at baseline, smoking history, and inversely related to serum AAT levels, which raises suspicion of a direct relationship between AATD and bronchiectasis. However, Low R2 values in our models suggested these variables only explained a small part of the variance in the dataset. Differences in age may be explained by the fact that bronchiectasis has a slow onset, and, therefore, those presenting to clinic later can be expected to have more advanced disease. The difference in smoking history would appear counterintuitive, but the analysis of EARCO found a similar pattern,18 and there is a well-established poorer prognosis of smokers in those with AATD.35,36
Bronchiectasis in AATD patients was not definitively proven to be related to lung function decline or exacerbation rate in this analysis. To our knowledge, no other studies have examined the effect of bronchiectasis on exacerbations in AATD cohorts. Like our study, the recent analysis of bronchiectasis in the EARCO database found no link with lung function, although these were static baseline measurements rather than the temporal changes assessed in the present study.18 A Portuguese team recently studied temporal lung function decline and also found no association with bronchiectasis diagnosis, although their study was limited to 43 patients.37 However, they found their patients with bronchiectasis to have more severe breathlessness as measured by mMRC score, findings replicated in our study. More severe symptoms, along with the nonassociation of bronchiectasis with COPD in our cohort, suggest the diagnosis of bronchiectasis in AATD may represent a clinically relevant endotype, and warrants specific assessment. While the finding of exacerbation rate being no higher in bronchiectatic patients was unexpected, there are several possible reasons this may have occurred, including mild disease, recall bias when reporting events at annual reviews, and different triggers to exacerbation. Consistent with the latter interpretation, preliminary microbiology data from the cohort shows that exacerbation events are not associated with expansion of prior colonizing organisms.38 Further research into characteristics of AATD exacerbations is, therefore, warranted.
One limitation of this study is its use of a historic database. While a significant proportion of COPD and bronchiectasis diagnoses have been assessed for accuracy, with low levels of errors, not all patients had spirometry and CT scans available for analysis. Ongoing data collection for our database now ensures spirometry is recorded, and CT scans are transferred from secondary care centers in digital format.
Milder disease is unlikely to have an impact on disease progression unless measured over very long time periods or with larger sample sizes, since in other causes of bronchiectasis only more severe dilatation, morphology, or more widespread disease have been observed to impact disease progression.27,39,40 In smaller sample sizes or shorter durations of follow-up, this will, therefore, reduce statistical power. Both these limitations are often present in research in AATD, due to its nature as a rare and slowly progressive disease.
Our cohort is one of the largest in Europe, which increases generalizability of our results, but despite this it only contains 239 bronchiectasis patients, not all of whom met our criteria for lung function decline assessment, leading to lower statistical power of regression analysis. However, within those who did have longitudinal data available, many were seen for over 5 years, as shown by the distribution of follow-up times, suggesting we should have captured slow progression. Finally, we did not have a contemporaneous, PiMM database for comparison of all characteristics in our study. This may be the subject of future research.
Conclusions
Bronchiectasis is present in a significant minority of PiZZ AATD patients, independently of concurrent COPD diagnosis. These patients are more likely to have more severe shortness of breath. Appropriate management of bronchiectasis in AATD is, therefore, essential. Without subcategorization, bronchiectasis does not predict lung function decline or exacerbation rate, but further study of radiological, microbiological, or biochemical subcategories of bronchiectasis may clarify this.
Acknowledgements
Author contributions: JDS is the guarantor of the manuscript, including the data and analysis. JDS undertook study design, data collection, and statistical analysis, and was the author of the first manuscript draft. PE assisted with statistical analysis, review of methodology and results, and edited manuscript drafts. MN assisted with study design, statistical analysis, and review of methodology and results. LR assessed CT scans for inter-rater reliability. AMT provided the initial study concept and reviewed the manuscript drafts. All authors reviewed and approved the final version of the manuscript.
Declaration of Interests
PE has received speaker fees from Chiesi, GSK, and AstraZeneca. AMT has had grants to her institution and/or honoraria from CSL Behring, Grifols, Vertex, Takeda, Chiesi, AstraZeneca, GSK, Sanofi, and Boehringer Ingelheim. JDS, MN, and LR have no conflicts of interest to declare.