Running Head: Effect of Multimorbidities in U.S. COPD Patients
Funding Support: The study was funded by Novartis Pharma AG.
Date of Acceptance: July 29, 2024 | Publication Online Date: August 5, 2024
Abbreviations: BMI=body mass index; CCI=Quan-Charlson comorbidity index; CDM=Optum’s de-identified Clinformatics® Data Mart Database; CFTR=cystic fibrosis transmembrane conductance regulator; CI=confidence interval; COPD=chronic obstructive pulmonary disease; DEXA=dual-energy x-ray absorptiometry; ED=emergency department; GERD=gastroesophageal reflux disease; HCRU=health care resource utilization; ICD-10-CM=International Classification of Diseases, 10th Revision Clinical Modification; ICS=inhaled corticosteroid; IL=interleukins; OCSs=oral corticosteroids; OR=odds ratio; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; PDE4=phosphodiesterase-4; POS=place of service; SABA=short-acting beta2-agonist; SAMA=short-acting muscarinic antagonist
Citation: Krishnan JK, Martinez FJ, Altman P, et al. Multimorbidities in COPD are associated with increased exacerbations and health care resource utilization in real-world patients from a U.S. database. Chronic Obstr Pulm Dis. 2024; 11(5): 472-481. doi: http://doi.org/10.15326/jcopdf.2024.0515
Online Supplemental Material: Read Online Supplemental Material (470KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex disease with both pulmonary manifestations and compounding systemic manifestations leading to multimorbidity.1 Multimorbidity has been shown to affect most patients with chronic obstructive airway diseases, including COPD,2 and can impact patients’ quality of life, frequency of exacerbation, and mortality.2-8 Pathophysiology underpinning multimorbidity in COPD is an area of ongoing investigation. Smoking is a shared risk factor for many chronic diseases, and physical inactivity, common in COPD, could contribute to multimorbidity.9 It has been suggested that accelerated aging, systemic inflammation, and altered immune cell function could all potentially play a role in the pathogenesis of morbidities in patients with COPD.10 The importance of multimorbidity in chronic diseases like COPD and the need for greater recognition, focus, and management of multimorbidity to improve health outcomes has been recognized.9,11 While a link between COPD and cardiovascular diseases is well established,12-14 we strived to examine less-studied comorbidities such as gastroesophageal reflux disease (GERD), diabetes, and osteoporosis. These diseases have recently been linked to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction and may have the potential to be addressed by systemic CFTR-related interventions.15 GERD in particular is highly prevalent in patients with COPD and is associated with increased exacerbations.16-18 A recent meta-analysis of data from 13,245 patients with COPD showed that GERD increased the risk of exacerbation (odds ratio [OR]: 5.37; 95% confidence interval [CI]: 2.71 to 10.64), and patients with COPD and GERD exhibited more exacerbations (weighted mean difference: 0.48; 95% CI: 0.31 to 0.65, albeit statistical heterogeneity and publication bias was acknowledged) than those with COPD alone.16
The objectives of this study were firstly to determine differences in the burden of multimorbidities in patients with COPD versus without COPD using real-world data; and secondly to determine the impact of 3 morbidities common in patients with COPD (GERD, diabetes, and osteoporosis) on exacerbations and COPD-related health care resource utilization (HCRU) in patients with COPD compared with those who have COPD but do not have the respective comorbidity.
Methods
Study Design
This was a retrospective, noninterventional cohort study based on the secondary use of deidentified data from Optum’s de-identified Clinformatics® Data Mart Database (CDM) (Supplementary Material in the online supplement). As a result, institutional review board or additional informed consent was not required.
The time period during which COPD and morbidity-related diagnoses were identified (identification period) was from January 1, 2016 to December 31, 2020. For the cohort of patients without COPD, the index date was defined as the first diagnosis of any kind, excluding COPD, in the identification period. The study period was from January 1, 2016 (when International Classification of Diseases 10th Revision Clinical Modification [ICD-10-CM] codes were introduced) to June 30, 2021 (Supplemental Figure S1 in the online supplement).
The follow-up period was defined as 5 years after the index date (inclusive) and patients were followed up until the end of the study period. If a patient died, or the period of continuous enrollment ended (allowing for gaps in continuous enrollment of ≤30 days), the earliest of these was considered as the end of follow-up. Five-year data are presented here.
Objective and Outcomes
The study compared the demographic and clinical characteristics of the defined matched COPD and non-COPD cohorts in the U.S. patient population. Proportions of morbidities of interest (GERD, diabetes, and osteoporosis) and overall morbidity burden represented by the Quan-Charlson comorbidity index (CCI) were compared between the COPD and non-COPD cohorts.
The main objective of this study was to compare risk, as determined by ORs, exacerbations, and COPD-related HCRU in the following groups: (1) patients with COPD and GERD versus patients with COPD but without GERD, (2) patients with COPD and diabetes versus patients with COPD but without diabetes, and (3) patients with COPD and osteoporosis/osteopenia versus patients with COPD but without osteoporosis/osteopenia.
Inclusion Criteria
Patients aged 40–89 years were included, and 2 main cohorts were defined as patients with COPD and a random 10% sample of the CDM population without COPD.
The inclusion criteria for patients with COPD were ≥2 diagnoses of COPD ≥30 days apart in their CDM record (i.e., outpatient and inpatient records) within the identification period and ≥12 months of active continuous enrollment in CDM from and including the index date. Inclusion criteria for patients without COPD were ≥1 medical claim with any valid ICD-10-CM diagnosis code excluding COPD, during the identification period and ≥12 months of active continuous enrollment in CDM from and including the index date. Use of ICD-10-CM diagnosis codes is a standard approach to identifying COPD in health systems.19 The disease subcohorts (COPD with/without GERD; COPD with/without diabetes; and COPD with/without osteoporosis/osteopenia) were defined as follows: “with” included patients with ≥2 diagnoses for the respective disease ≥30 days apart during the identification period; and “without” included patients with no diagnoses for that disease during the 5-year follow-up time period. ICD-10-CM codes for diagnoses are shown in Supplemental Table S1 in the online supplement. Patients with a concurrent asthma diagnosis were included. Please see Supplementary Material in the online supplement for exclusion criteria.
Matching
All cohorts were matched 1:1 with their comparator group (i.e., patients with COPD versus patients without COPD) in order to minimize bias within the comparison. All cohorts were matched to their comparator group using simple age-matching based on decades (to maximize the number of patients in each cohort). Weight status and the presence of “underweight” status (based on ICD-10-CM code of R63.4 [abnormal weight loss] or R64 [cachexia]) were used for matching of patients with COPD and diabetes and patients with osteoporosis/osteopenia, respectively. See Supplementary Material in the online supplement for further details.
Analysis
Descriptive statistics (mean, standard deviation, n, proportion) for demographic and clinical characteristics (age, sex, weight status), CCI, COPD exacerbations, and COPD-related HCRU were generated.
Severe COPD exacerbations were defined as a hospitalization or emergency department (ED) admission with a primary diagnosis of COPD. A primary diagnosis was defined as having a relevant ICD-10-CM code for COPD (J41.x, J42.x, J43.x, J44.x) in the diagnosis table in diagnosis position “01”. Moderate COPD exacerbations were defined as an outpatient visit with any diagnosis for COPD with ≤2 prescriptions of oral corticosteroids (OCSs) within 7 days of the outpatient visit, or ≥1 prescription of antibiotics or anti-infective agents within 7 days of the outpatient visit. The upper limit for the duration of OCSs/ antibiotic/anti-infective agent prescription was set at 30 days to ensure longer durations of OCSs were captured. Longer duration of corticosteroid therapy is typically 7 days and over.20,21 A sensitivity analysis was also performed for severe COPD exacerbations where these were defined as a hospitalization admission with a primary diagnosis for COPD. Having any COPD exacerbations was defined as having at least one moderate COPD exacerbation or one severe exacerbation. As the duration of COPD exacerbations can vary,22,23 if 2 exacerbations happened within 14 days, they were merged into one exacerbation.24 If one exacerbation was moderate and one was severe, the merged exacerbation was categorized as severe.
COPD-related HCRU included COPD-related inpatient hospitalizations and ED visits. HCRU is indicated by at least one claim with any diagnosis of COPD and the given HCRU location (hospital or EDs). The HCRU type (i.e., ED versus inpatient) was determined based on Centers for Medicare and Medicaid Services place of service (POS) codes25 using the following algorithm: (1) ED visits defined as records with POS = 23, and (2) hospitalizations, defined as records of non-ED visits, with non-missing confinement ID.
Conditional logistic regression models were used to compare rates of the outcomes of interest (COPD exacerbations and HCRU) in different cohorts at 5 years; ORs, 95% CIs, and p-values are reported. Outcome and independent variables are described in the Supplementary Material in the online supplement. Models were not adjusted for further covariates. ORs were calculated for presence of comorbidities of interest (GERD, diabetes, osteoporosis), COPD exacerbations, and COPD-related HCRU during the 5-year follow-up period.
Medication Use
Descriptive statistics were reported for medication use, as classified by the Global initiative for chronic Obstructive Lund Disease (GOLD) 2023 report,26 for each index year and up to 5 years after and including the index date. See Supplementary Material in the online supplement for further details.
Results
Patient Flow and Demographics
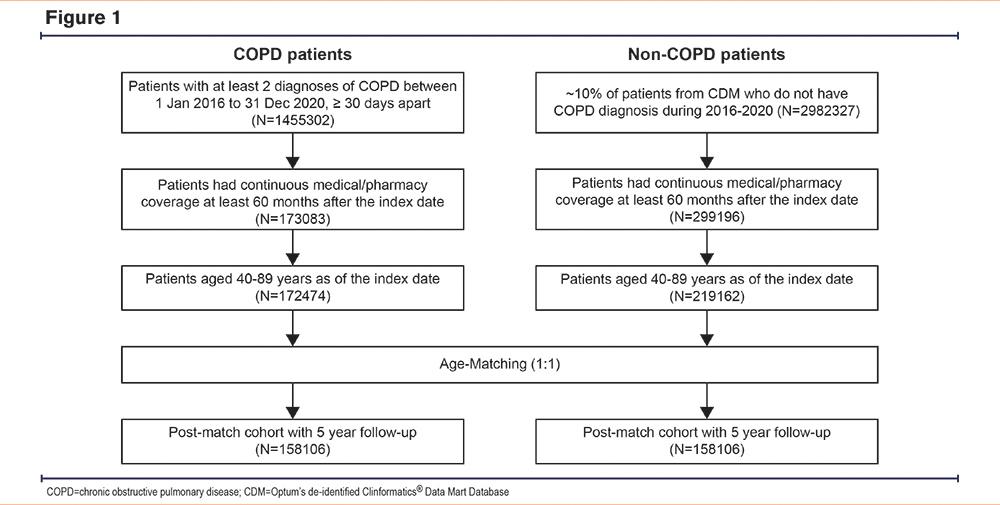
In total, 172,474 patients were identified as belonging to the COPD cohort. After age-matching, a COPD cohort of 158,106 patients and a corresponding non-COPD cohort of 158,106 patients were identified (Figure 1).
Patient Characteristics
The mean (standard deviation) age at index date in the COPD cohort was 71.09 (8.95) years compared with 70.88 (9.11) years in the age-matched non-COPD cohort (Table 1). A total of 55.7% of patients in the COPD cohort were female compared to 58.5% of patients in the age-matched non-COPD cohort. The proportions of patients with GERD, diabetes, and osteoporosis diabetes were higher amongst the COPD cohort than the age-matched non-COPD cohort.
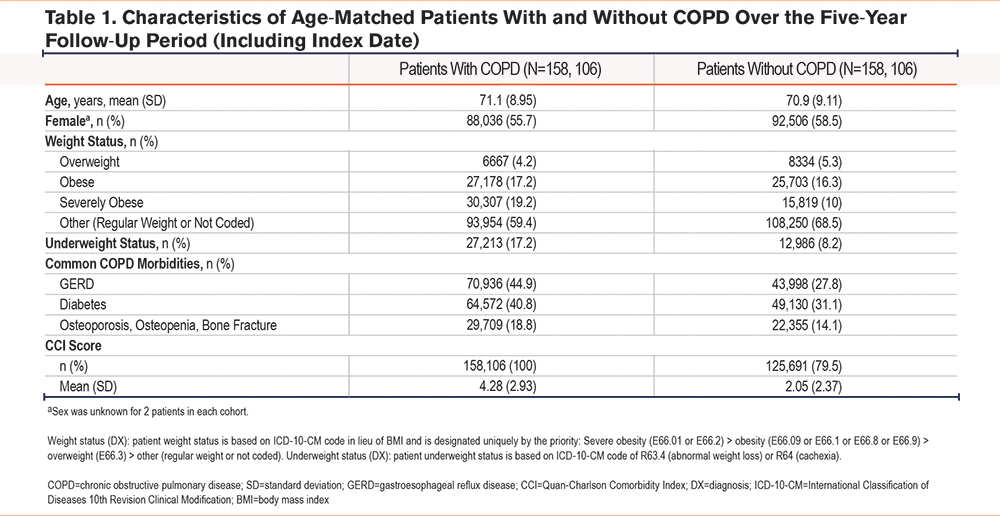
Higher proportions of patients in the COPD cohort were categorized as either underweight or severely obese and had diagnoses of selected common morbidities compared with the age-matched non-COPD cohort during the 5-year follow-up period (Table 1). Patients in the COPD cohort had a higher mean CCI score than patients in the age-matched non-COPD cohort (Table 1).
Exacerbations
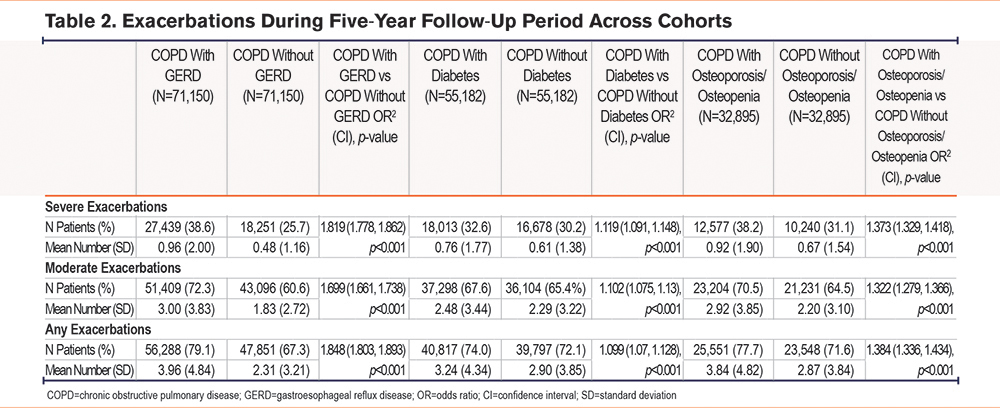
In the COPD cohort, patients with GERD had a higher risk of severe exacerbations (OR [95% CI]: 1.819 [1.778, 1.862], p<0.001), moderate exacerbations (OR [95% CI]: 1.699 [1.661, 1.738], p<0.001), and any exacerbations (OR [95% CI]: 1.848 [1.803, 1.893], p<0.001), than age-matched COPD patients without GERD (Table 2). Patients with COPD and diabetes were also at increased risk of severe exacerbations (OR [CI]: 1.119 [1.091, 1.148], p<0.001), moderate exacerbations (OR [CI]: 1.102 [1.075, 1.13], p<0.001), and any exacerbations (OR [CI]: 1.099 [1.07, 1.128], p<0.001) than those age- and weight-matched without diabetes (Table 2). Similarly, COPD patients with osteoporosis/osteopenia had higher risks of severe, moderate, and any exacerbations than age- and underweight status-matched COPD patients without osteoporosis/osteopenia (OR [95% CI]: 1.373 [1.329, 1.418], p<0.001; OR [95% CI]: 1.322 [1.279, 1.366], p<0.001, and OR [95% CI]: 1.384 [1.336, 1.434], p<0.001, respectively) (Table 2). Sensitivity analysis revealed lower numbers of severe exacerbations by a small margin when ED visits were not included in the severe exacerbation definition (Supplementary Table S10 in the online supplement).
COPD-Related Health Care Resource Utilization
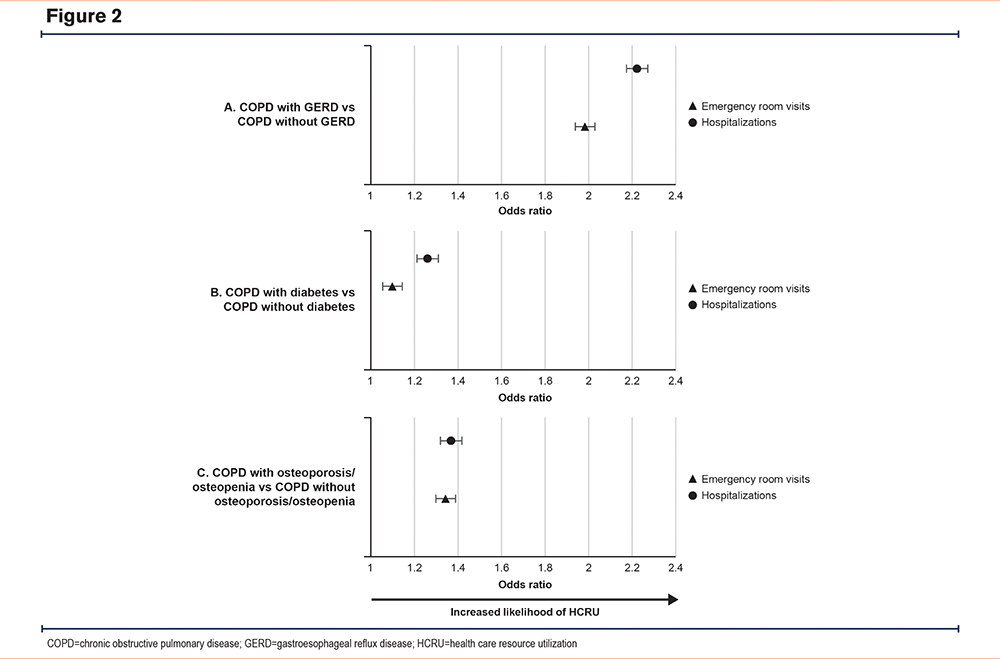
In all cases, patients with COPD and selected morbidities (GERD, diabetes or osteoporosis/osteopenia) had an increased risk (OR>1) of COPD-related HCRU compared with the matched cohort with COPD without the selected morbidities (Figure 2, Supplementary Tables S4–S6 in the online supplement).
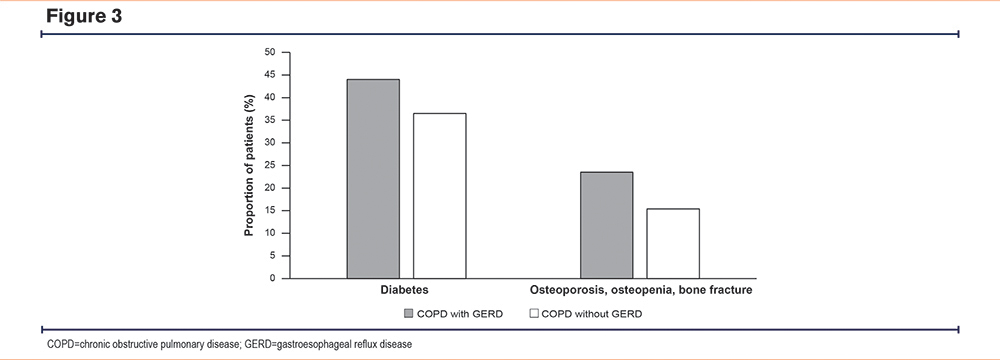
Multiple Morbidities
A higher proportion of patients with COPD and GERD also had diabetes or osteoporosis/osteopenia when assessed against patients with COPD only (Figure 3). As expected, COPD patients with GERD, diabetes, or osteoporosis/osteopenia had high CCI scores, and more of these patients experienced additional morbidities than their matched cohort without GERD, diabetes, or osteoporosis/osteopenia, respectively (Supplementary Table S3 in the online supplement). Patients with COPD and GERD were more at risk of having diabetes or osteoporosis/osteopenia than those age-matched patients with COPD without GERD (OR [95% CI]: 1.366 [1.337, 1.396], p<0.001 and OR [95% CI]: 1.706 [1.66, 1.753], p<0.001, respectively). Patients with COPD and diabetes had a higher risk of GERD and a lower risk of having osteoporosis/osteopenia than those age- and weight-matched patients with COPD without diabetes (OR [CI]: 1.223 [1.194, 1.252], p<0.001 and OR [95% CI]: 0.7147 [0.6928, 0.7373], p<0.001, respectively). Similarly, patients with COPD and osteoporosis/osteopenia had a higher risk of GERD and a lower risk of diabetes than those with age and underweight status-matched patients with COPD without osteoporosis/osteopenia (OR [95% CI]: 1.662 [1.61, 1.715], p<0.001 and OR [95% CI]: 0.6493 [0.6287, 0.6707], p<0.001, respectively).
Medication Use
Respiratory medication use in the matched cohorts over the course of 5 years is shown in Supplementary Tables S7-S9 in the online supplement. Patients with COPD and GERD or osteoporosis/osteopenia had generally higher medication use, including inhaled corticosteroid (ICS)-containing therapies, than patients in their matched cohort without those morbidities. In contrast, patients in the COPD with diabetes cohort used fewer medications than their age-matched cohort without diabetes (Supplementary Tables S7-S9 in the online supplement). Despite their high ICS use (Supplementary Table S7 in the online supplement), patients with GERD and patients with osteoporosis/osteopenia (Supplementary Table S9 in the online supplement) were at higher risk of exacerbations than their matched cohorts with COPD only (Table 2).
Discussion
We demonstrate that within our age-matched cohort analysis of a patient population from the CDM, those identified as having COPD had more comorbid conditions and higher CCI scores than those without COPD. Furthermore, this analysis showed that the cohort of COPD patients who had comorbidities such as GERD, diabetes, and osteoporosis/osteopenia, experienced increased risk of both severe and moderate exacerbations and COPD-related HCRU in this study compared with the matched COPD-only cohort. These findings from real-world data support the results of previous studies showing that diabetes, GERD, and osteoporosis are comorbidities frequently observed in patients with COPD and that they impact the risk of exacerbation.2-7 COPD is associated with other diverse morbidities and while there may be distinct pathways of clinical expression of these morbidities, e.g., metabolic syndrome27 or osteoporosis/sarcopenia,28 the common factor between most extrapulmonary manifestations of COPD is chronic systemic inflammation.29
Even though patients with GERD or osteoporosis/osteopenia had generally higher medication use than those without these conditions, high exacerbation risk is still observed for these cohorts. There are few options currently available for COPD treatment beyond inhaled therapies with bronchodilatory and anti-inflammatory effects, that could address systemic manifestations of COPD.30 More recent development programs have introduced systemic treatment approaches, e.g., with injectable biologics and oral small molecules.31,32 Research is ongoing utilizing biologics directed against interleukins (IL-5, IL-4, IL-13, IL-33) or their receptors as well as antithymic stromal lymphopoietin.33 CFTR, which is found throughout the body and expressed in a range of cell types, is also a potential target.34-37 CFTR dysfunction can be genetic, as is the case in cystic fibrosis, or may be acquired, for example, as a result of smoking.38,39 Moreover, GERD, diabetes, and osteoporosis are common in patients with CFTR dysfunction,40-42 and CFTR potentiators have been shown to ameliorate clinical symptoms and improve lung function in patients with COPD and chronic bronchitis.37,43-45 Future research is needed to evaluate potential therapies that target both local and systemic manifestations of COPD.
While confirmation of our findings in other datasets would be beneficial, a strength of this study is the breadth of the CDM; having a large number of patients in each cohort allowed for appropriate matching and up to 5 years of longitudinal follow-up. There are limitations to using CDM, including the limitations inherent in any claims-based dataset, such as incomplete, inaccurate, or missing data, or lack of specific billing codes for some conditions.46 Our methodology helps analyze COPD beyond the classical spirometry markers for disease by employing a diagnosis code approach; however, an ICD-based approach can also lead to misclassification/omission. Claims data were used to identify comorbidities and while these claims were verified and adjudicated prior to inclusion in the database, it is possible that patients with the 4 investigated conditions (COPD, GERD, diabetes, osteoporosis/osteopenia) did not have a corresponding diagnosis because of coder accuracy or limited clinical context. Accuracy of codes can vary depending on the knowledge of the coder. Coding practices may vary across different health care settings and providers. One limitation of the use of diagnostic codes to identify osteoporosis/osteopenia in patients with COPD relates to the fact that these conditions are undetected without dual-energy X-ray absorptiometry (DEXA) screening and DEXA screening is not considered in our analysis meaning some patients with osteoporosis may not have been detected. Consequently, it is not possible to tell which patients have had a DEXA screen and which have not or whether this has been the determinant for their diagnosis code. This is a limitation as many patients with COPD may be referred for DEXA screening in relation to their exposure to systemic steroids for treatment of COPD exacerbations. Furthermore, the cohort with diabetes had less inhaled steroid use than the matched cohort without diabetes, which may have impacted the number of moderate exacerbations recorded from the cohort based on the definition used. The matching process was not changed for observations after March 2020, i.e., the impact of the COVID-19 pandemic on patient records was not considered. It is possible that the broad definition of COPD-related HCRU, where any ED admission or hospitalization with any diagnosis of COPD is considered COPD-related, might have led to an overestimation of COPD-related HCRU in our study. Finally, spirometry data were not included in our analysis and were not considered for patient matching, therefore, we are unable to determine the severity of the COPD in those patients with multiple morbidities with higher rates of HCRU and exacerbation.
While the molecular mechanisms behind the systemic components of COPD are not well understood, our results demonstrate that morbidities like GERD, diabetes, and osteoporosis are very common in patients with COPD and that patients with COPD who have one of these morbidities are at greater risk of exacerbations and HCRU. It has long been known that the presence of additional morbidities impacts COPD progression and outcomes and that high resource use and costs are associated with COPD and multiple morbidities.47,48 The current view of COPD is of a multicomponent disease with many clinical phenotypes, systemic manifestations, and additional associated morbidities.49 With the advent of novel therapies that target systemic components of COPD, it will be important to assess their efficacy beyond the lungs. There are no current COPD treatments which address extrapulmonary morbidities and, therefore, there is an urgent unmet need for novel therapies that address key underlying pathologies and reduce the disease burden for patients with COPD.
Acknowledgements
Data Sharing: The data used were licensed from Optum and are not publicly available.
Author contributions: All authors meet criteria for authorship in accordance with the International Committee of Medical Journal Editors. The authors take responsibility for the integrity of the data and analysis and the work as a whole. Editorial support to draft and revise the manuscript based on input from all authors was provided by Sorcha Mc Ginty, PhD, and Ian Wright, PhD (Novartis). All authors contributed to revisions for critically important content. All authors approved this submitted version of the manuscript.
Financial support for medical editorial assistance was provided by Novartis Pharma AG, Basel, Switzerland. We thank Sorcha Mc Ginty, PhD, and Ian Wright, PhD (Novartis Medical and Knowledge Solutions, Global Business Solutions, Dublin, Ireland), for their medical editorial assistance with this manuscript. We thank Justin Gomme for his contribution to the study plan development.
Declaration of Interests
JK declares Medical Writing and Editorial Services funded by Novartis; grants to institution from the American Thoracic Society Fellowship in Health Equity; Research Assistance for Primary Parents Award; COMMUNITY Center Investigator Development Core; National Institutes of Health (NIH) T32 HL134629; Weill Cornell Fund for the Future Award; consulting fee for the 2023 Verona Pharma advisory board; medical writing support for a different manuscript from Novartis; medication samples delivered to the institution from Boehringer-Ingelheim and GlaxoSmithKline; donor gift to institution from the Donna Redel Research Fund and Thomas King Fellowship. FJM declares Novartis as sponsor of the parent study; receipt of payment or honoraria for disease state presentation, grant support and consulting fees from AstrazZeneca, AstraZeneca is a partner in the SPIROMICS program and National Heart, Lung, and Blood Institute (NHLBI) CAPTURE validation study; receipt of consulting fees and in kind grant support from Chiesi, Chiesi is a partner in the SPIROMICS program; participation in data safety and monitoring board and receipt of payment or honoraria for disease state presentation, consulting fees and grant support from GSK, GSK is a partner in the SPIROMICS program and NHLBI CAPTURE validation study; receipt of consulting fees and in-kind grant support from Sanofi/Regeneron, Sanofi/Regeneron are partners of the SPIROMICS program; payments made to the COPD Foundation for partnership in SPIROMICS and/or CAPTURE from NHLBI - AstraZeneca, Chiesi, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Polarean, Sanofi/Regeneron, Sunovion, and TEVA Pharmaceuticals; receipt of consulting fees from Boehringer Ingelheim, Boehringer Ingelheim is a partner of the SPIROMICS program and partners in the NHLBI CAPTURE validation study; receipt of consulting fees from CSL Behring and Pulmonx; receipt of consulting fees from Novartis, Polarean, Sunovion and Teva (all partners of the SPIROMICS program); receipt of consulting fees from Theravance/Viatris, Theravance/Viatris are partners in the NHLBI CAPTURE validation study; receipt of payment or honoraria for COPD CME from UpToDate; participation in event adjudication committee for Medtronic. PA declares ownership of Novartis stock and was an employee of Novartis at the time of writing this article. VLFB is an employee of Novartis. EK, RP and MS are Novartis employees and own Novartis stock. HK declares ownership of Novartis stock and was an employee of Novartis at the time of writing this article; honoraria from De Gruyter GmbH for her role as Editor-in-Chief of the journal Epidemiologic Methods and for her role as cochair of the ISPOR Society Special Interest Group on Statistics in Health Economics and Outcomes Research.