Running Head: Digital Health and COPD Health Care Utilization
Funding Support: This study was funded by Desert Oasis Healthcare, Boehringer Ingelheim, Propeller Health, a subsidiary of ResMed, and ResMed.
Date of Acceptance: August 19, 2024 | Publication Online Date: September 6, 2024
Abbreviations: COPD=chronic obstructive pulmonary disease; ED=emergency department; HCRU=health care resource utilization; IDN=integrated delivery network; OCS=oral corticosteroid; PSM=propensity score matching; QI=quality improvement; SMD=standardized mean difference; SD=standard deviation
Citation: Brazeal T, Kaye L, Vuong V, Le J, Peris Z, Barrett MA. Reducing health care resource utilization in COPD: a retrospective matched control analysis of a digital quality improvement program. Chronic Obstr Pulm Dis. 2024; 11(5): 515-523. doi: http://doi.org/10.15326/jcopdf.2024.0532
Online Supplemental Material: Read Online Supplemental Material (375KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is an obstructive lung disease characterized by shortness of breath and a persistent, productive cough. Globally, COPD is the third leading cause of death and estimated to impact 250 million adults today,1,2 with prevalence expected to increase by 23% in the next 30 years.3 In the United States today, approximately 32 million adults live with COPD, of which only half have received an official diagnosis.
The first-line pharmacological treatment for COPD includes maintenance and short-acting beta agonist inhalers. Patients may also be prescribed antibiotics and oral corticosteroids (OCSs) should their symptoms worsen to an acute exacerbation state. Despite these treatments, approximately 16% of people with COPD experience a worsening of symptoms, or an exacerbation necessitating an emergency department (ED) visit and/or hospitalization.4 Further, 1 in 5 discharged patients with COPD will be readmitted within 30 days.5
In the United States, annual costs6,7 for treating COPD are estimated at $50 billion/annum and estimated to grow to $60.5 billion/annum by the year 2029. Costs are primarily driven by exacerbations requiring ED visits and hospitalizations, and thus, reduction of acute exacerbations is not only a key therapeutic target in the management of COPD but also an economic one.8
As such, digital health interventions have become an increasingly promising option to help promote improved management of COPD. Digital health encompasses a diverse panel of tools to support remote patient monitoring, such as wearables, medication sensors, smartphone apps, and/or short messaging services. Such tools have been shown to benefit those living with COPD, promoting increased adherence to daily maintenance inhalers,9 reducing disease burden, and promoting better symptom management.10,11 Digital health can also mitigate health care access issues, allowing patients to monitor their condition and communicate with their health care providers via telemedicine options and electronic messaging services.12 Studies also suggest that such digital interventions could significantly reduce health care resource utilization (HCRU),13 potentially reducing health care-related costs in COPD.14
Despite the demonstrated value of digital health in COPD, few studies have explored clinically integrated digital workflows and their impact on COPD HCRU in a robust manner using real-world claims data.12 As such, we conducted a 6-month retrospective matched control analysis to assess the impact of a digital quality improvement program, delivered by clinical pharmacists, on HCRU among people living with COPD.
Methods
Design and Setting
This study is a retrospective propensity score-matched control analysis of a digital quality improvement program offered to members of a large integrated delivery network (IDN) in southern California (Desert Oasis Healthcare, Palm Springs, California).
Through the IDN, over 60,000 members receive acute and long-term medical care and wellness services. For members with chronic conditions like COPD, available services include a team of clinical pharmacists who provide further virtual and in-person clinical oversight, aiming to promote better clinical outcomes and quality of life through improved access to care and national guideline-based approaches. All members enrolled through the IDN receive an initial assessment visit with a clinical pharmacist to review medical and medication history, and to develop a medication plan that manages both clinical and member needs. Following this initial assessment, clinical pharmacists continue to provide remote oversight of member medication needs and remain available for consultation as desired by the member.
In the quality improvement program analyzed in this study, members with COPD had standard access to the IDN care system and were also offered by their clinical pharmacist access to a Food and Drug Administration-cleared digital platform for COPD self-management (Figure 1; Propeller Health, Madison, Wisconsin). The digital platform included medication sensors to capture the time and date of inhaler use, and a smartphone app for medication reminders, feedback, and education. The app also prompted members to complete the COPD Assessment Test. Clinical pharmacists had access to a clinician dashboard with relevant member medication use data, which was used to inform follow-up care, such as ordering follow-up tests, adherence interventions, and medication adjustments or early intervention for worsening symptoms including prescribing inhalers, OCSs, and antibiotics as indicated.10,15

Recruitment and Enrollment
Clinical pharmacists identified eligible members based on the following inclusion criteria: COPD diagnosis, ≥40 years of age, and a system-compatible maintenance and/or rescue inhaler. Members were excluded if they: (1) had a history of other chronic respiratory diagnoses or illnesses (cystic fibrosis, lung cancer, history of pneumothoraces); (2) were pregnant; (3) had comorbid end-stage renal disease; (4) were on dialysis; (5) were receiving end of life care; (6) were unable to use the digital platform; or (6) did not accept the digital platform’s Terms of Services.16 Eligible members were invited to enroll in the digital quality improvement program between November 2016 and November 2018.
The quality improvement program was reviewed by the WCG Institutional Review Board and was determined to be exempt from further review (June 2021 #1-1442257-1).
Outcomes
The primary objective of this study was to assess HCRU for all-cause and COPD-related causes at 6 months post-enrollment, using a retrospective propensity score-matched control analysis. HCRU was defined as one or more of the following: prescription of an OCS, prescription of a respiratory-related antibiotic, ED visit, and/or a hospitalization. Secondary analyses examined changes in office and urgent care visits over the same time period. Further detail, including the International Classification of Diseases-10th Revision codes used to develop the COPD-related causes, are included in the online supplement.17,18
Covariates
The study considered a comprehensive set of covariates, categorized into demographics, neighborhood socioeconomic status, clinical characteristics, and baseline HCRU.
Demographics included age at index date, gender, race, and primary language. Neighborhood affluence score included zip code-level composite indices (income, education, and employment rates) and the number of internet providers per 1000 residents.
The neighborhood affluence score data was obtained from the National Neighborhood Data Archive.19 The index was linked to the study cohort using residential zip codes converted to zip code tabulation areas. It aimed to provide a broader understanding of the socioeconomic environment of the participants by averaging 3 census indicators, including: proportion of households with income greater than $75,000 U.S. dollars, proportion of population age 16+ employed in professional or managerial occupations, and proportion of adults with a bachelor's degree or higher. Scores range from 0 to 1.0, with higher scores indicating higher levels of affluence.
Clinical characteristics examined insurance type, smoking status, and the number of comorbid conditions. Baseline HCRU was measured for both all-cause and COPD-related services in the 6 months preceding the index date. This included all-cause office visits, urgent care visits, ED visits, hospitalizations, and COPD-related urgent care visits, ED visits, hospitalizations, as well as prescription fills for COPD-related antibiotics and OCSs.
Analysis
Index Date Determination
For the digital quality improvement group, the index date was designated as the first synchronization date of the digital device. If the gap between the first synchronization date and the first medication use date exceeded 60 days, the latter was used as the index date. For the control group, index dates were randomly assigned to each individual to match the distribution of the quality improvement group's index dates, ensuring comparative analysis.
Verification of the index date included ensuring the index date preceded any recorded date of death. Patients were required to have 6 months of continuous HCRU data pre- and postindex date.
Propensity Score Matching (PSM)
Observable baseline differences between those in the digital quality improvement program and those in the control were accounted for using PSM. To accomplish the matching, we first calculated a propensity score—the likelihood of being in the digital quality improvement program—using a probit regression model on the covariates described above. We then tested various matching algorithms and evaluated which one achieved the best balance in the observed covariates using multiple approaches, including love plots, histograms, and standardized mean difference (SMD), with |SMD|<0.1 considered indicative of good balance.20 The balance diagnostics indicated that optimal full matching with a caliper of 0.2 achieved the best balance compared to other approaches (nearest neighbor matching, optimal pair matching, genetic matching, and inverse probability of treatment weighting). Additionally, the year of the index date was specified in the matching algorithm as the variable on which to match to ensure similar timeframe of HCRU between the 2 groups. Optimal full matching was performed using the MatchIt package21 in R, which calls functions from the optmatch package.22
Descriptive statistics were employed to present baseline characteristics of the study population using the Chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Differences in HCRU outcome between the matched populations were compared with weighted Wilcoxon rank-sum tests, using the “survey” package (v4.1-1) in R.23 A sensitivity analysis using either adjusted weighted Poisson or adjusted weighted negative binomial models, depending on if overdispersion was present, was conducted to account for matching imbalance. We first ran Poisson models for each of the outcomes, we then tested for overdispersion using the R “performance” (v0.10.2) to determine whether a Poisson or negative binomial model better suited the data. The final model, either Poisson or negative binomial, was incorporated with the full matching weights using the R packages “survey” and “sjstats” (v0.18.2), respectively. Cluster-robust standard errors accounting for both the matching weights and pair membership were estimated. Postmatching imbalanced covariates were included in the models. Analyses were conducted using R statistical software version R version 4.1.1.
Results
Of the 23,725 IDN members who were considered for analysis, 13,658 met analytic inclusion criteria (79 members were included in the quality improvement group and 13,579 in the matched control group) (Consort Flow table in the online supplement). Overall, matched members were on average male, White, and over 70 years of age (Table 1).
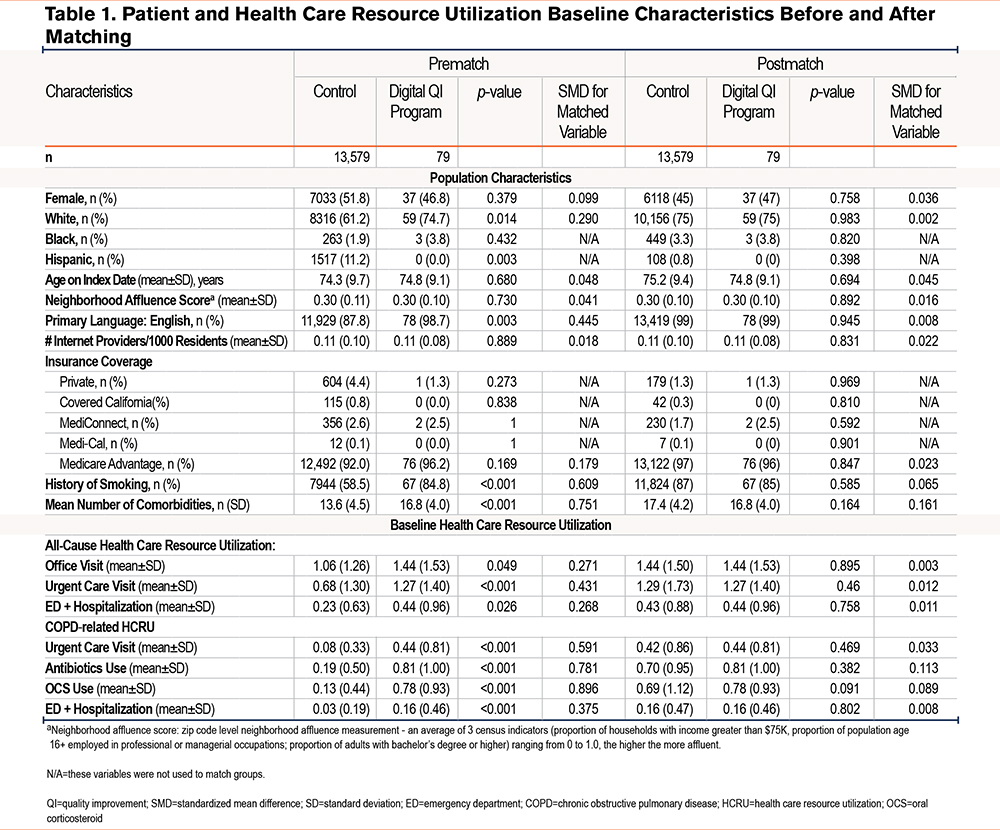
Postmatch, an acceptable SMD (|SMD|<0.1) was achieved for all relevant baseline demographics, except for the number of comorbidities, which was slightly higher in the matched control group. Similarly, an acceptable postmatch SMD was achieved for HCRU, except for COPD-related antibiotic use, which was slightly higher in the digital quality improvement group. (Table 1, Supplement Figure 2s in the online supplement)
Postindex HCRU
At 6 months postindex, there were fewer all-cause ED visits and hospitalizations among those in the digital quality improvement program compared to those in the matched control (0.25±0.63 versus 0.47±1.09 events, p=0.059). There were also 66.7% relatively fewer COPD-related ED visits and hospitalizations in the digital quality improvement program compared to matched controls (0.04±0.19 versus 0.12±0.44, p=0.044).
More all-cause and COPD-related urgent care visits were observed in the digital quality improvement group at 6 months compared to those in the matched control group (all-cause: 1.41 versus 1.11, p=0.017; COPD-related: 0.30 versus 0.14, p=0.027). All-cause office visits were also slightly higher in the digital quality improvement program compared to the matched control, but the difference did not reach statistical significance (p=0.398). Finally, there was a larger proportion of COPD-related antibiotics and OCS prescription fills observed in the digital quality improvement program compared to matched controls, but only differences in fills for OCS prescriptions reached statistical significance (p=0.045) (Table 2, Figure 2).
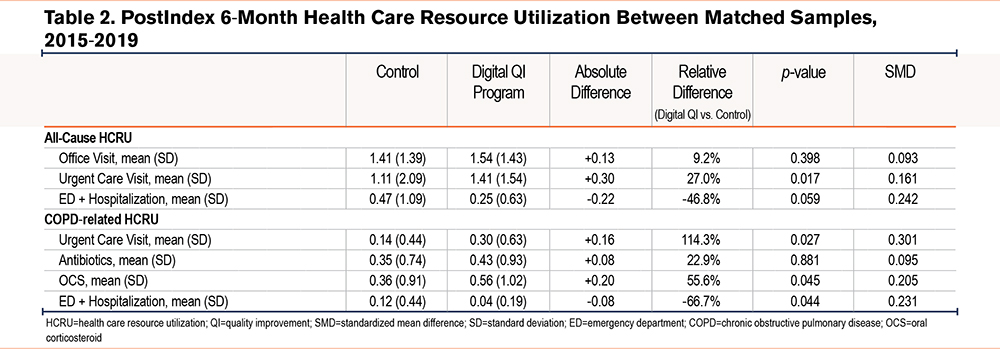
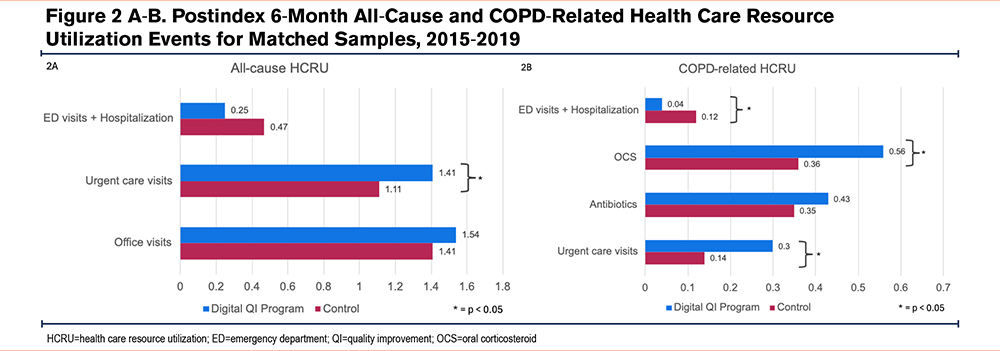
Sensitivity analyses to account for matching imbalances for both comorbidity count and antibiotic fills revealed similar trends in HCRU outcomes (Table 3s in the online supplement).
Discussion
This study aimed to assess the impact of a digital quality improvement program, delivered by clinical pharmacists, on HCRU in COPD. Compared to matched controls at 6 months, participants in the digital quality improvement program saw a nearly two-thirds relative reduction in COPD-related ED visits and hospitalizations (p=0.044), as well as a 47% reduction in all-cause ED visits and hospitalizations (p=0.059). Concurrently, participants in the quality improvement program also had higher rates of COPD-related prescription fills for antibiotics and OCSs, as well as a greater number of nonacute care visits compared to matched controls. Based on a U.S. Medicare population, the reduction in ED/hospitalizations translates to annual estimated cost-savings of up to $308,319 per 100 patients ([1.3333 fewer ED visits/hospitalizations/month per 100 patients] × 12 months × $19,270/hospitalization),24 notwithstanding consideration for program implementation costs and reimbursement codes.14
This real-world study is one of the first to better understand how a clinically integrated digital program for COPD self-management influences both acute and nonacute health care utilization. Interestingly, a consistent reduction in acute events (e.g., ED visits and hospitalizations) in the digital QI group was paired with an increase in COPD-related prescriptions and utilization in more primary care settings. This shift from acute to primary care utilization may reflect better condition management, self-awareness, and timely clinical intervention, potentially mitigating exacerbations necessitating an ED visit or hospitalization. Notably, we also observed an increase in the number of urgent care visits in the quality improvement program. The IDN considered an urgent care visit similarly to an unscheduled clinic visit and represented a more proactive and cost-efficient alternative to ED visits and hospitalizations.
While the observed reduction in COPD-related ED visits and hospitalizations aligns well with existing digital health literature and its impact on HCRU outcomes,25 a common critique of prior studies has been the reliance on relatively small samples and single-arm observations. Thus, our study sought to match intervention members with a large pool of control members, robustly matching on 16 criteria, aiming to mitigate such criticisms and further building confidence in the outcomes observed. Further, the use of IDN data allowed for a more complete data assessment, capturing most health care interactions.
These promising findings underscore the value of a digital self-management program in COPD, while also highlighting the emerging role of clinical pharmacists in chronic disease management.26 In the United States, clinical pharmacists play a central role in COPD management not only for medication dispensing but also for patient oversight to ensure that prescribed medications are meeting the needs of the patient. As such, digital health integration within clinical pharmacist workflows may better support the role of the clinical pharmacist through regular access to medication usage behaviors and early warning alerts for patients with worsening symptoms.
Our study, while robust in its findings, is not without limitations. First, the study was a retrospective analysis of real-world claims data and thus, inherent internal and external biases exist. While we attempted to mitigate these biases by including a robustly matched control group, we were unable to reach perfect group balance for the number of comorbidities and antibiotic prescriptions. Follow-up sensitivity analyses, however, did confirm similar observed results. Secondly, while our study was limited to a 6-month assessment period to increase sample size, it is possible that a longer assessment period would have better accounted for confounding factors like seasonal variations. Thirdly, our sample was comprised primarily of older White males, and thus, generalizability to other populations may be limited. Future research should explore the health care impact of digital COPD interventions in underserved and disadvantaged populations, which have historically poorer access to health care facilities and higher rates of acute care visits.27 Our study was reliant on claims data and may have missed other relevant patient interactions such as telehealth visits; however, patients within the IDN sought care almost exclusively through the IDN network. Further, claims data is typically used for administrative purposes and not research, thus, the accuracy of the data should be understood considering its limitations.17 Finally, we were unable to examine other lifestyle management programs that may have impacted the results observed. In COPD, pulmonary rehabilitation and smoking cessation programs have both demonstrated promising clinical and lifestyle outcomes for patients and future studies should consider exploring these contextual variables.
Conclusion
This study highlights the value of integrating digital health tools within existing clinical workflows and the crucial role of clinical pharmacists in enhancing patient care. Our findings suggest that implementing digital health solutions within an integrated delivery network not only offers a scalable approach to managing COPD, but also can help promote more oversight and reduce costly emergency department visits and hospitalizations.
Acknowledgments
Author contributions: TB, LK, VV, JL, and MAB were responsible for study design. ZP and VV were responsible for data collection and cleaning and VV conducted study analyses. LK, MAB, and VV completed the first manuscript draft. All authors were responsible for reviewing and editing the final manuscript.
Data sharing statement: The data that support the findings of this study are not publicly available.
Other acknowledgments: We thank Vanessa Di Cataldo for her writing support.
Declaration of Interest
TB, JL, and ZP are employees of Desert Oasis Healthcare. LK, VV, and MAB are employees of ResMed, the parent company of Propeller Health.