Running Head: Escalations from Home Monitoring in COPD
Funding Support: The study was funded by Spire Health.
Date of Acceptance: September 18, 2024 | Publication Online Date: October 2, 2024
Abbreviations: AECOPD=acute exacerbation of chronic obstructive pulmonary disease; BMI=body mass index; CLs=clinical liaisons; COPD=chronic obstructive pulmonary disease; EMRs=electronic medical records; ED=emergency department; FEV1=forced expiratory volume in 1 second; GOLD=Global initiative for chronic Obstructive Pulmonary Disease; ICD-10=International Classification of Diseases-Tenth Revision; IRB=institutional review board; RPM=remote physiologic monitoring; SD=standard deviation
Citation: Teresi RK, Hendricks AC, Moraveji N, Murray RK, Polsky M, Maselli DJ. Clinical interventions following escalations from a continuous respiratory monitoring service in patients with chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2024; 11(6): 558-568. doi: http://doi.org/10.15326/jcopdf.2023.0475
Online Supplemental Material: Read Online Supplemental Material (231KB)
Background
Chronic obstructive pulmonary disease (COPD), the third leading cause of death globally,1 is “a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, expectoration, exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.”2 COPD is punctuated by acute exacerbations of COPD (AECOPDs), a sudden worsening of symptoms, which contribute significantly to disease progression and acute health care utilization.
Although earlier treatment of AECOPDs has been shown to reduce their severity and associated acute care utilization,3 care-seeking is often delayed. The reasons for delay are complex and multifactorial, including symptom variability and a lack of awareness of changes in symptoms associated with AECPODs, unwillingness to burden caregivers and providers with potential false alarms, and a desire to minimize the potential significance of changes in symptoms.4-6
A primary goal for optimal health care delivery in COPD is implementing integrated care, but it often implies greater demand on health care resources because of the need for heightened care coordination.7 This involves information transfer, assessment, monitoring, follow-up, and facilitating transitions across care settings.
Therein lies the potential of leveraging technologies enabling remote clinical professionals to monitor, triage, assess, and interact with patients to improve provider efficiency while minimizing unnecessary burden on providers and patients.8 Such an approach may employ some combination of remote monitoring, algorithmic interpretation of physiologic trends, patient-facing digital interfaces, virtual consultation, health coaching, virtual rehabilitation, and others.
Recent advancements in remote physiologic monitoring (RPM) technologies have shown considerable promise in transforming integrated care for COPD. RPM technologies in COPD, which currently encompass a range of commercially available wearable devices (e.g. wristbands,9,10 armbands,11 vests,12,13 and rings14), have been increasingly studied for their ability to collect real-time physiological data.15 The integration of artificial intelligence and machine learning algorithms into RPM technologies has begun to offer sophisticated analyses of collected data, increasing the predictability of AECOPDs and facilitating proactive clinical evaluation and intervention.16-18 However, research is warranted to systematically evaluate this potential in real-world settings.19,20
While conceptually appealing, implementing integrated care through RPM technologies in the real world comes with its challenges, such as ensuring long-term adherence among a population that has varying levels of motivation and technological literacy as well as ensuring that equitable access to such technologies is provided. While many of the components of integrated care have been evaluated to meet care goals in COPD,21-24 the outcomes of escalations from RPM services, where the RPM service prompts a patient to be seen by their provider, have not been reported upon.
The present study evaluates the implementation of a continuous respiratory monitoring service which includes clinical triage that may escalate patients for care from their pulmonary care provider. The service has been previously shown to be associated with reduced acute care utilization.21 While it was hypothesized that the mechanism for this reduction was more timely outpatient intervention resulting from the service’s acute escalations to care providers, no data were provided as to the actions that providers took in outpatient visits during the study period.
This present study aims to address this gap by reporting how frequently COPD patients seen by their pulmonary care provider after being escalated by an RPM service are treated in a manner consistent with them having experienced an AECOPD, providing a comprehensive report of outpatient visits that took place within 7 days of escalations. The results may also help clinicians understand the implications of escalations from remote monitoring on provider and patient burden by evaluating whether providers deemed the resulting outpatient visits clinically necessary. To do this, we employed a framework consistent with prior work22,23 where treatment-based definitions of COPD exacerbations are used to evaluate the outcomes and clinical relevance of medical escalations.
Methods
Study Design
This was a retrospective, observational, real-world study of COPD patients at a multi-office pulmonary practice situated in a metropolitan area in the mid-Atlantic region of the United States. An independent institutional review board (IRB; Western IRB #00000533) approved the study and granted a waiver for documentation of informed consent, given that acquiring such consent would have been impractical and the study entailed no more than minimal risk.
Inclusion Criteria
The patients in this analysis had previously elected to enroll in the service as part of their clinical management. All patients were active at the partnering practice, had been clinician-diagnosed with COPD, and carried at least one of the following International Classification of Diseases-Tenth Revision (ICD-10) classification system codes: COPD (J44), emphysema (J43), or chronic bronchitis (J42). They were also continuously enrolled for a period of at least 12 months as of May 1, 2022 and had at least one escalated office visit while enrolled.
Intervention
The intervention consisted of a service with 3 components: (1) continuous cardiorespiratory monitoring, (2) algorithmic notification of physiologic deterioration, and (3) clinical liaisons who engage patients, respond to algorithmic notifications, triage patients, and “escalate” patients by notifying their provider of the need for further assessment (Figure 1).
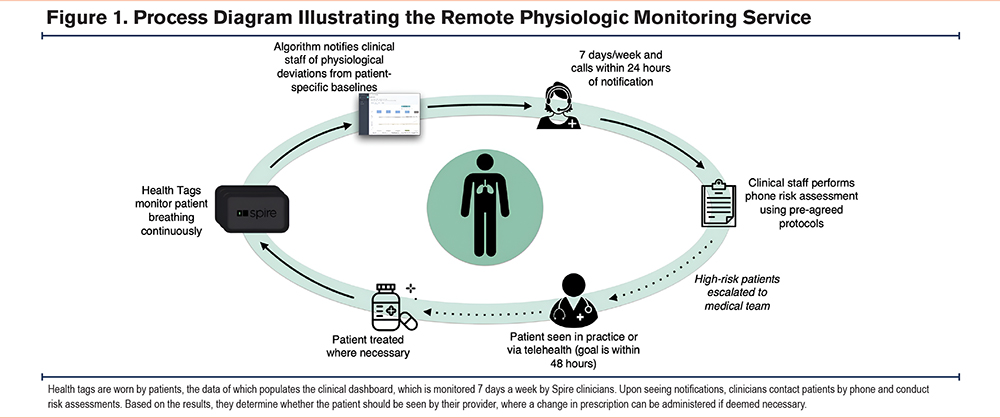
Continuous Monitoring
Physiologic monitoring was done through a U.S. Food and Drug Administration-cleared, proprietary device called a health tag (Wellinks; New Haven, Connecticut), which included sensors for respiratory force (respiration), photoplethysmography (pulse), and tri-axis accelerometers (physical activity or steps). The health tag was designed to minimize patient burden and social stigma. Patients adhered the health tag to the inner waistband of their undergarment (Figure 2).

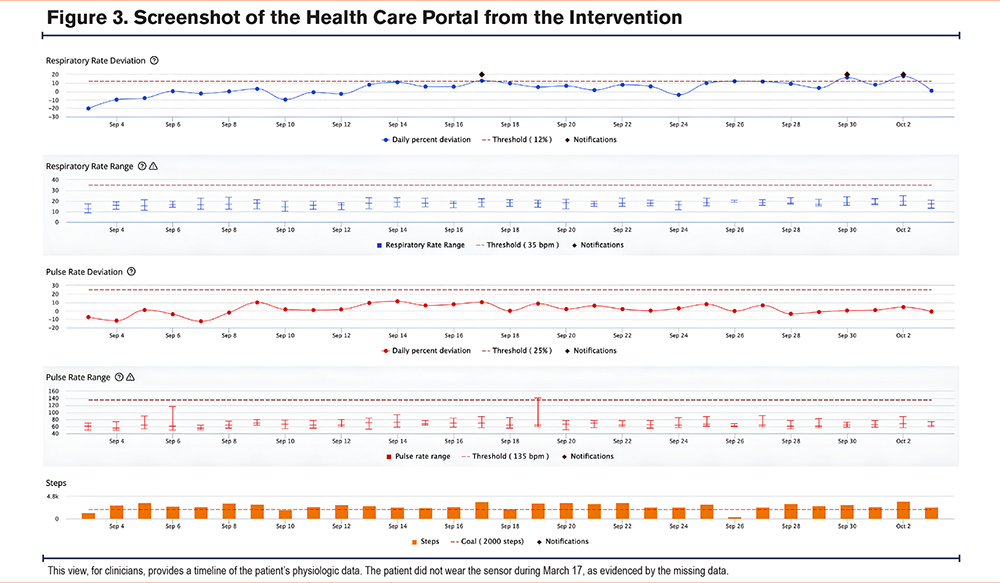
Each patient was provided with 6 health tags, one for each of the patient’s undergarments. If a patient requested more health tags, they were given more, up to 8. They are engineered to last over a year as a set without recharging and are for prolonged skin contact. The respiration and accelerometer sensors operate continuously while the pulse sensor takes readings every 4 minutes. Health tag data are relayed via a dedicated in-home hub to a virtual clinical dashboard shown in Figure 3 and used by a dedicated team of the service’s clinical liaisons (CLs).
Algorithmic Notifications
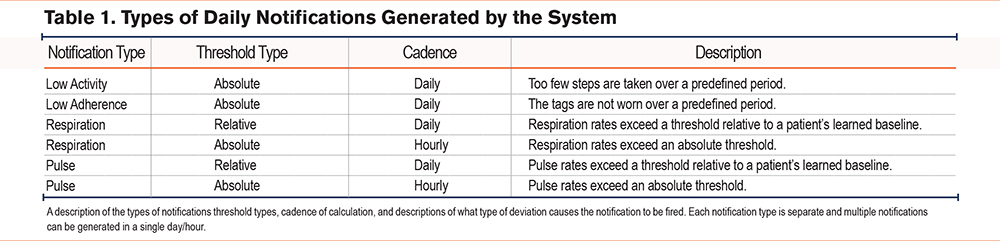
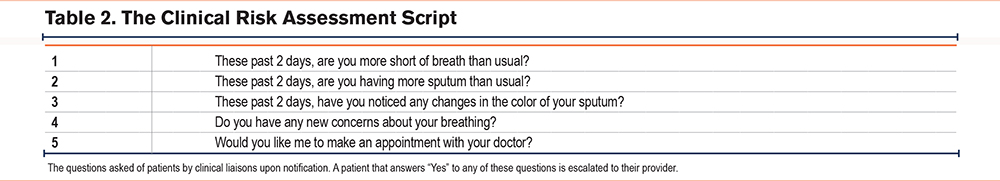
Algorithm-driven notifications were displayed on the clinical dashboard when health tag data indicated deviation from predetermined thresholds. Notifications were triggered by nonadherence (i.e., health tags not worn for at least 8 hours in a day for a predefined duration), inactivity (i.e., too few steps taken within a predefined duration), and relative or absolute increases in pulse and respiration rates (Table 1). Within 24 hours of the occurrence of any notification (48 hours on weekends), a CL called the patient to conduct a risk assessment (Table 2).
Integrated Clinical Liaisons
The integrated clinical liaison team, which consisted of respiratory therapists and nurses experienced in respiratory disease, reviewed algorithmic notifications and data in the dashboard. Though they could have been third-party clinicians, the CLs in this analysis were employed by the RPM provider and worked 8am–5pm during the weekdays, with a CL on call available on weekends to address critical respiratory and pulse rates. Upon notification of potential patient deterioration, a CL (usually assigned to that patient) contacted the patient by phone to conduct the standardized clinical risk assessment to assess changes on relevant symptoms.
When a patient failed the risk assessment by answering “yes” to one or more of its questions or they could not be reached after 3 attempts over 48 hours, they were escalated: notifying a patient’s provider to recommend that their patient be evaluated. Multiple notifications could precede an escalation in the case that a patient exceeded multiple physiologic thresholds before failing a risk assessment. Multiple escalations could precede an escalated office visit in the case that escalations persisted, and a patient had yet to be seen by their provider.
The service CLs had access to the partnering practice’s emergency medical records (EMRs), where they could schedule either in-person or virtual office visits with the patient’s consent. During an escalation, a patient could choose to forego scheduling an office visit. Regardless, a one-page summary of their recent data trends was posted in the EMR for their provider’s review. This summary report included the CL’s notes from the phone call, a patient’s response to the risk assessment, and a timeline of physiologic data. This workflow is illustrated in Figure 1.
In the absence of notifications, they conducted a monthly “check-in” call to address any potential patient concerns, support self-management and patient education, and perform a routine patient assessment. While physical activity was monitored by the health tags, and the day’s step count was shown to patients, neither explicit activity coaching nor virtual pulmonary rehabilitation was utilized during the observation period.
Data Coding
Manual chart reviews of the practice’s EMRs were performed on escalated office visit records created between the dates of May 2020 and May 2022. Office visits that occurred more than 7 days following an escalation were excluded.
Clinical interventions during office visits were grouped into 6 categories: an increase or change in oral corticosteroids, antibiotics, or inhaled medications; referral for further testing or imaging; other symptomatic treatment; and referral to the emergency department (ED) (Table 3). An office visit was determined to include treatment consistent with the management of an AECOPD if at least one of the codes included corticosteroids, antibiotics, or inhaled medications.
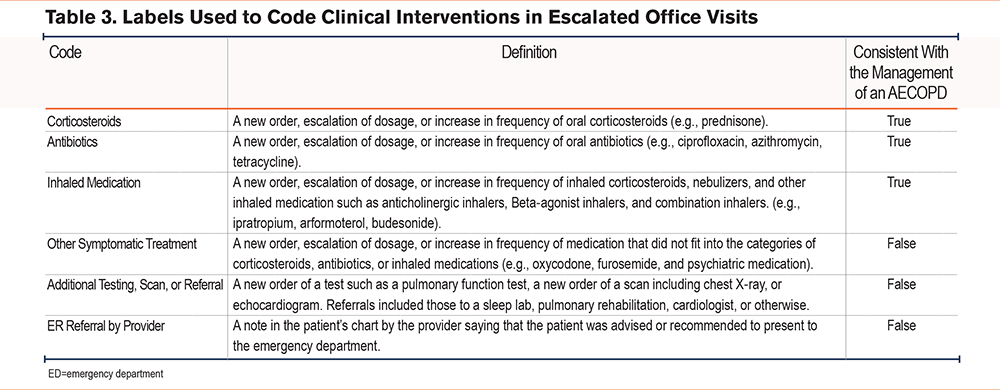
For this analysis, notifications were tied to escalations if they occurred in the 3 days preceding the escalation. We denoted escalations caused by failing a risk assessment during a call within 3 days after a notification as “notification-based” or, otherwise, “check-in–based.”
Results
Demographics
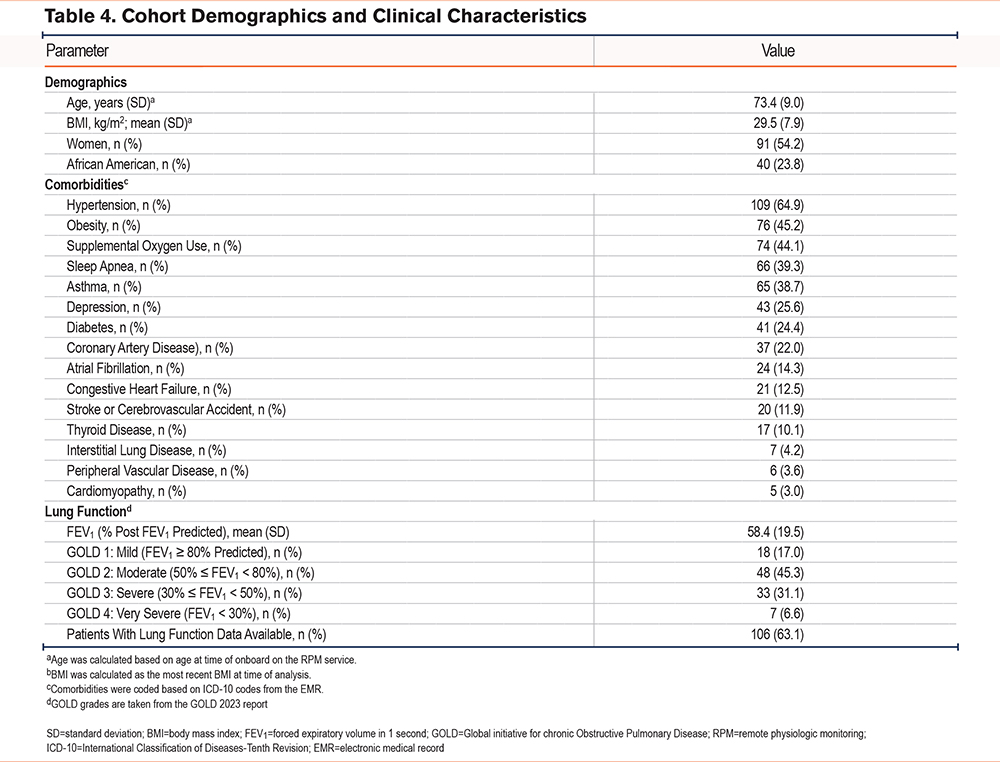
The cohort consisted of 168 COPD patients, the majority women (54.2%), with a mean age of 73.4 years (SD: 9.0). African Americans comprised 23.8% of the cohort. The cohort exhibited a broad spectrum of comorbidities with a high prevalence of hypertension (64.9%), obesity (body mass index ≥ 30; 45.2%), and asthma (38.7%). The cohort was generally comprised of moderate to severe COPD patients, averaging a postbronchodilator forced expiratory volume in 1 second (FEV1) of 58.4% (SD: 19.5%) of predicted, with 83.0% of the cohort having an FEV1 below 80% of predicted and 37.7% having an FEV1 below 50% of predicted (Table 4).
Enrollment and Adherence
Across the study period, patients were enrolled for an average duration of 17.5 (standard deviation [SD] 3.2) months. Health tags were worn for at least 8 hours per day on 76.7% of days during the study period (the 8-hour threshold was chosen based on the notification algorithm), with a mean and median of 16.0 (SD: 9.3) and 21.8 hours worn per day, respectively. On average, patients completed 1.5 (SD: 0.3) calls with a CL per month.
Notifications and Escalations
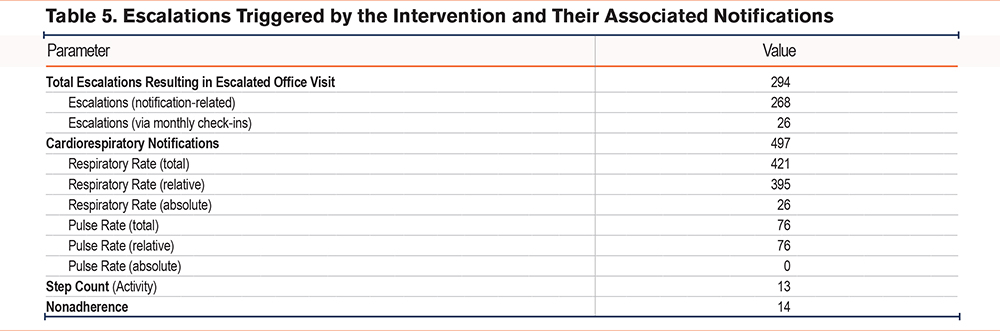
The study recorded 294 escalations that were associated with office visits. A total of 26 (8.8%) were triggered during monthly check-in calls and the remaining 268 (91.2%) by one or more of 524 notifications. Of these notifications, 497 (94.8%) were cardiorespiratory. Of these, 421 (84.7%) were respiratory (26 absolute and 395 relative) and 76 (15.3%) were pulse (all relative). A total of 27 (10.1%) of the notification-based escalations included both respiratory and pulse notifications in the prior 3 days. In addition to cardiorespiratory notifications, there were 14 (2.7%) notifications for adherence and 13 (2.5%) activity notifications observed (Table 5).
Office Visits
There were 245 escalated office visits in total, with virtual office visits occurring more frequently (153, 62.4%) than in-person office visits (92, 37.6%). These office visits were overseen by 33 distinct providers (advanced practitioners or physicians); the mean duration from escalation to office visit (time to visit) was 2.9 (SD: 2.2) days.
Of the escalated office visits, 163 (66.5%) resulted in treatment consistent with the management of an AECOPD: 116 (47.3%) were coded for “corticosteroids,” 81 (33.1%) were coded for “antibiotics,” and 64 (26.1%) were coded for “inhaled medication.” Four (1.6%) visits were for “ED referral by the provider,” 94 (38.4%) were coded for “additional testing, scan, or referral,” and 31 (12.7%) were coded for “other symptomatic treatment” (see Figure 4 and Table 6).
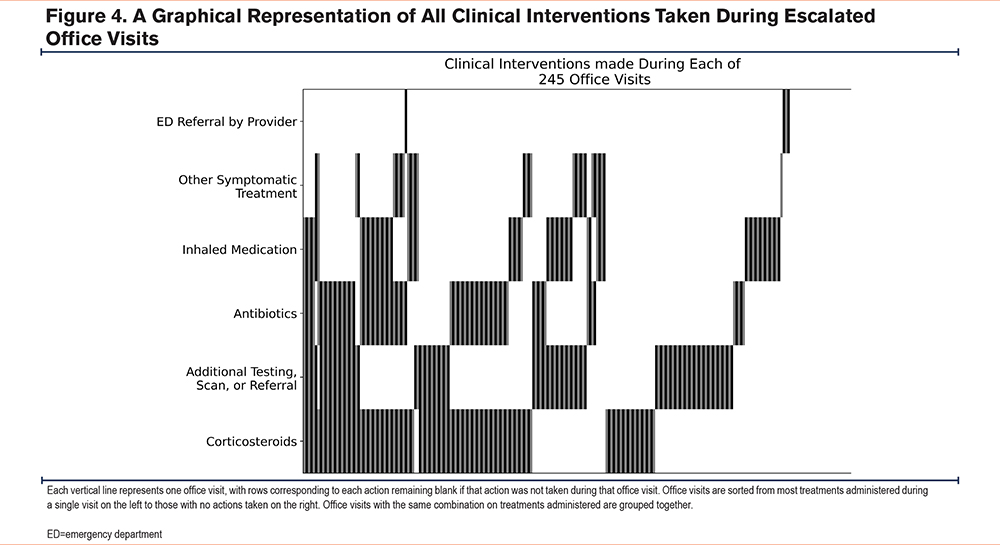
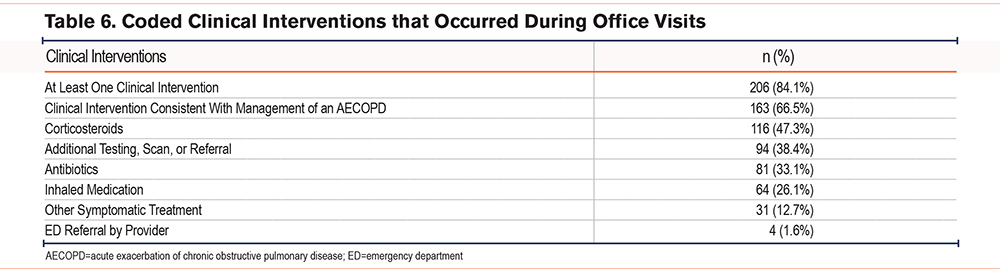
Overall, 206 (84.1%) visits were coded for at least one clinical intervention, with 78 (31.8%) being coded for one, 79 (32.2%) being coded for 2, 42 (17.1%) being coded for 3, and 7 (2.9%) being coded for 4 clinical interventions in the same visit.
All 4 instances in which providers referred patients directly to the ED resulted in patients presenting there; 3 of these resulted in hospital admissions. The primary ICD-10 codes for these ED visits were acute on chronic respiratory failure with hypoxia (J96.02), COVID-19 pneumonia (U07.1), and acute COPD exacerbation (J44.1).
Discussion
We present a detailed description of clinical interventions triggered by escalations from a continuous respiratory monitoring and clinical triage service. Of the escalated office visits, 84.1% resulted in clinical intervention and 66.5% in treatment consistent with the management of an AECOPD. Changes in respiratory rate were the dominant cause for notifications, escalations, and associated clinical interventions (Supplementary Table S1 in the online supplement), mirroring the symptomology of COPD and the high prevalence of related respiratory comorbidities in the analyzed population (Supplementary Table S2 in the online supplement). The clinical interventions that were most highly associated with each other were a prescription for corticosteroids and for antibiotics, which is in line with a typical intervention for an AECOPD (Supplementary Table S3 in the online supplement).
A relatively small proportion (1.6%) of escalated office visits resulted in referral to the ED. It is assumed that these were severe exacerbations. This small proportion lends support to the hypothesis that the system generally identified patients early enough to prevent severe deterioration.
In addition to accurate and prompt clinical detection, remote monitoring services require efficient clinical workflows and expedited clinical assessment for interventions to be swiftly administered. Patients must heed escalations from the service by choosing to go to the office in a timely manner, underscoring the importance of patient engagement in a remote monitoring setting. The observed time to visit of 2.9 days was less than half the average 6–7 day care-seeking delay reported in another study of COPD patients who were on a care management intervention.24 Furthermore, in another study, the time between symptom onset and treatment was a median of 3.69 days, with 40.1% of exacerbations unreported.3 This supports the hypothesis that escalations from the service removes obstacles in patient decision-making and enables faster access to care.
Because of the retrospective nature of the study, the 33 providers represented in this analysis were unaware of the study’s analytical objectives or goals, bolstering the integrity of the study’s results. Access to the practice’s EMRs by the platform’s CLs allowed for streamlined triaging of escalated patients to their pulmonologist, exemplifying the promise of integrating such platforms into EMR systems and allowing our study to evaluate a mature RPM implementation.
The findings reinforce the potential for this type of service to enable early detection and timely intervention, which can lead to decreased severity of exacerbations, reduced need for acute care, and more efficient resource allocation. Due to the service’s goal of identifying deterioration early enough to prevent the need for hospital admission, it is expected to escalate a significant proportion of patients who do not warrant additional intervention. However, a majority of escalations resulted in clinically meaningful actions, supporting the claim that providers found the visits to be clinically relevant. Further research is warranted to fully understand and quantify the potential impact of such platforms on clinical workflow, provider satisfaction, acute care utilization, and successful disease management among COPD patients.
These results have inherent limitations. Though we identified certain interventions during escalated office visits as being consistent with the management of an AECOPD, we lack data to confirm this assessment due to the limitations of clinical documentation. Due to the absence of a suitable control or comparison group, we cannot conclude that a patient being escalated reduced their expected time-to-visit nor can we compare the results to interventions at nonescalated office visits. Thus, we cannot employ a rigorous causal framework to enable us to definitively attribute these office visits to corresponding escalations. However, we can conclude that service-triggered escalations were associated with actionable medical interventions, which is its purported goal.
Other limitations include that the study did not evaluate patients who declined to see their provider upon escalation. Additionally, providers’ knowledge of when office visits were scheduled via escalations may have influenced their decisions regarding intervention. Finally, this study assessed only one service as described above and was implemented at only a single site.
The goal of this analysis was to evaluate the clinical interventions during office visits triggered by the monitoring service in a COPD cohort. In doing so, the current study contributes to the burgeoning RPM literature by offering a novel evaluation framework for COPD respiratory monitoring programs.15,21,25 We highlight our method to evaluate the impact of the intervention, given the high barriers to conducting large-scale randomized control trials.
These findings support a purported mechanism for remote monitoring to improve patient outcomes, showing that providers are highly likely to intervene in a patient’s care following an escalation of care. This contributes to the body of evidence in support of using remote monitoring for the purpose of facilitating early medical intervention, promoting proactive disease management, and enabling advanced clinical decision support systems. In addition, future research should focus on optimizing these systems to capitalize on continuous monitoring data for efficient COPD patient management at scale and improve the risk stratification of patient populations. Large-scale implementation studies are needed to study the cost-effectiveness of these systems and how clinical workflows can be adapted to maximize respiratory monitoring integration and effectiveness at diverse sites, leading to improved patient outcomes and optimized health care delivery.
Conclusions
In a cohort of COPD patients enrolled in a continuous respiratory monitoring service, provider encounters following clinical escalations from the service often resulted in their provider administering a treatment to the escalated patient; treatments administered were consistent with the patient experiencing an AECOPD 66.5% of the time. This finding supports the claimed mechanism for remote monitoring to improve patient outcomes through the timely identification and treatment of AECOPDs.
Acknowledgements
Author contributions: AH, RT, and NM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MP, NM, AH, RT, and RM contributed to the study design and concept. RT, AH, and NM drafted the manuscript. All authors contributed to interpreting the data and editing the manuscript. NM and MP supervised the study. All authors approved the final version of the submitted manuscript.
Data sharing statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
We acknowledge and thank all the study participants and the providers who treated the patients.
Declaration of Interests
AH, RT, NM, and RM are employees of Spire Health. MP is a consultant to Spire Health. DM has no conflicts of interest to declare.