Running Head: RESP-FIT: A COPD Muscle Strength Training Intervention
Funding Support: This study was funded by the National Institutes of Health National Institute of Nursing Research (NIH/NINR) award number P20NR016575 and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Numbers UL1RR029882 and UL1TR000062. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NINR.
Date of Acceptance: September 16, 2024 | Publication Online Date: October 2, 2024
Abbreviations: BMI=body mass index; CI=constant interval; COPD=chronic obstructive pulmonary disease; EMA=ecological momentary assessment; EMST=expiratory muscle strength training; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; IMST=inspiratory muscle strength training; M=mean; MCID=minimal clinical important difference; mMRC=modified Medical Research Council; PEmax=maximal expiratory pressure; PImax=maximal inspiratory pressure; PROMIS=Patient-Reported Outcomes Measurement Information System; PROs=patient-reported outcomes; QoL=quality of life; RESP-FIT=RESPiratory FITness program; RMST=respiratory muscle strength training; SEMCD-6=Self-Efficacy for Managing Chronic Disease 6-item scale; SD=standard deviation; SDT=Self-Determination Theory; SGRQ=St George’s Respiratory Questionnaire
Citation: Miller SN, Mueller M, Nichols M, et al. RESP-FIT: a technology-enhanced combined inspiratory and expiratory muscle strength training intervention for adults with COPD. Chronic Obstr Pulm Dis. 2024; 11(6): 569-581. doi: http://doi.org/10.15326/jcopdf.2024.0523
Online Supplemental Material: Read Online Supplemental Material (257KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease associated with substantial functional morbidity, activity-limiting symptoms, and respiratory muscle weakness.1,2 One of the most common and distressing COPD symptoms is dyspnea, also known as breathlessness, air hunger, or shortness of breath. Causes of dyspnea are multifactorial, often triggered by increases in respiratory load, dynamic hyperinflation, peripheral muscle dysfunction, declines in lung function, and physical deconditioning.3-5 Dyspnea is a powerfully aversive sensation, and many patients with COPD learn to fear and avoid activities such as exercise that may induce dyspnea. Disease-related resistance and/or obstruction from COPD, plus associated reductions in physical activity, contribute to physical deconditioning and reduced respiratory muscle strength, negatively impacting both upper and lower airway functions.
Respiratory muscle strength training (RMST) is an empirically validated therapy known to increase respiratory muscle strength and airway defenses in healthy adults, athletes, and patient populations with degenerative neurological and respiratory diseases such as COPD.6 RMST is performed with a portable training device tailored to individual inspiratory capacity. By applying respiratory force (inhaling or exhaling) needed to surpass a pressure threshold, respiratory and upper airway muscles are forced into a state of “overload” that improves respiratory muscle strength and coordination over time. Enhanced mechanical efficiency leads to decreased ventilatory demand and relief of breathlessness,4 and can be measured through the sampling of maximal inspiratory and expiratory pressures (PImax, PEmax) via a handheld manometer. RMST programs typically include either inspiratory muscle strength training (IMST) or expiratory muscle strength training (EMST) which strengthens PImax and PEmax respectively. IMST improves muscle strength in COPD, however, clinical results including effects on exercise tolerance, symptoms of dyspnea and fatigue, and health-related quality of life are inconsistent.7 By combining IMST and EMST, both inspiratory and expiratory muscles are challenged to maximize overall respiratory muscle strength training.8 While few studies9-11 have evaluated the combined use of combined IMST and EMST in COPD, findings suggest potential increases in PImax and PEmax with moderate improvement of dyspnea. However, previous studies used recall to track adherence and effects on patient-reported outcomes (PROs) and symptoms other than dyspnea are unknown. Dyspnea recall is often unreliable and may not be reflective of accurate severity.12-14
mHealth
As symptoms and airflow limitations change frequently in COPD, day-to-day disease management is dependent upon individual self-management and symptom recognition. Technology can facilitate individual disease management and quality of life using refined, interactive interventions that complement (not replace) current pulmonary rehabilitation.15-17 Smartphone or mobile technology (mHealth) can be used to self-manage physical activity18 especially after hospital discharge19-21 and for ecological momentary assessment (EMA). EMA is the capture of symptoms in real-time in the home environment, and provides insight into symptom trends over time, reducing recall bias and improving ecological validity.14,22-24 To our knowledge, there have been no studies that have explored the effects of a technology-enhanced combined IMST and EMST respiratory muscle strength training intervention or monitored symptoms over time using EMA.
Intervention Development
The RESPiratory FITness (RESP-FIT) program is a theoretically-grounded, 6-week, technology-enhanced RMST intervention consisting of 5 training days per week using a combined inspiratory and expiratory muscle strength training device with added Bluetooth-enabled frequency and asynchronous video feedback.25,26 The Self-Determination Theory (SDT) served as the foundational framework for both intervention development and the measurement model. SDT concepts are predictive of self-management regulation, and useful for framing and guiding intervention development.27 RESP-FIT was iteratively designed using concepts of the SDT, including autonomy (feeling empowered and having a choice), competence (feeling capable and effective), and relatedness (feeling connected to others). The platform was subsequently refined with repeated stakeholder (patients, families, clinicians) input.25,28-30 Symptoms are reported and captured as they occur, using EMA via smartphone technology.26 We have previously reported that the mHealth platform is acceptable and study procedures (recruitment, enrollment, and retention) are feasible,25 but have not reported on effects of the 6-week RESP-FIT intervention on respiratory muscle strength, symptoms, and patient-reported outcomes. For hypothesis-generating purposes, we aimed to explore whether effects on respiratory muscle strength, general disease self-management, and symptom domains supported carrying out future appropriately powered studies. Thus, the purpose of this manuscript is to report on the generated hypotheses and exploratory aims of the RESP-FIT intervention including symptoms, patient-reported outcomes, and respiratory muscle strength.
Materials & Methods
Sample and Setting
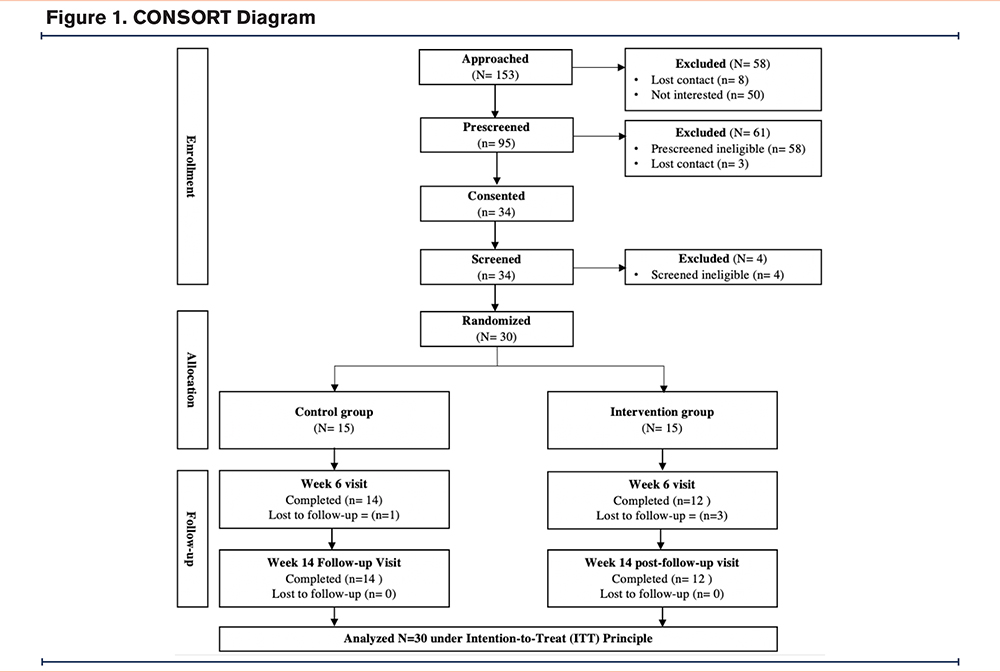
This study was conducted in the outpatient setting of a large academic medical center in Charleston, South Carolina. Prior to recruitment and enrollment, institutional review board approval (Pro00071706) was obtained, and the study was registered in ClinicalTrials.gov (NCT03652662). Adults over the age of 40 years with moderate to severe COPD (spirometry values: forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] <0.7 and FEV1% predicted < 50%), a dyspnea score ≥ “1” on the modified Medical Research Council (mMRC) questionnaire, and the ability to read and write in English were included. Exclusion criteria were: (1) pregnancy or less than 1-year postpartum, (2) diagnosed cognitive deficit or observed lack of understanding during the informed consent process, (3) mobility impairment that would impair the ability to participate in the intervention, (4) lack of cellular phone service or WiFi access, and (5) unwillingness to wear physical activity tracker daily, follow protocol, or attend study visits. This study was conducted in accordance with the Declaration of Helsinki. A total of 30 participants were recruited using a combination of convenience and snowball sampling, provider referrals, flyers, and direct contact25 across a combination of urban, rural, and medically underserved areas (Figure 1). Informed consent from participants was obtained either in-person or via Research Electronic Data Capture31 e-Consent per individual personal preference.
Procedures
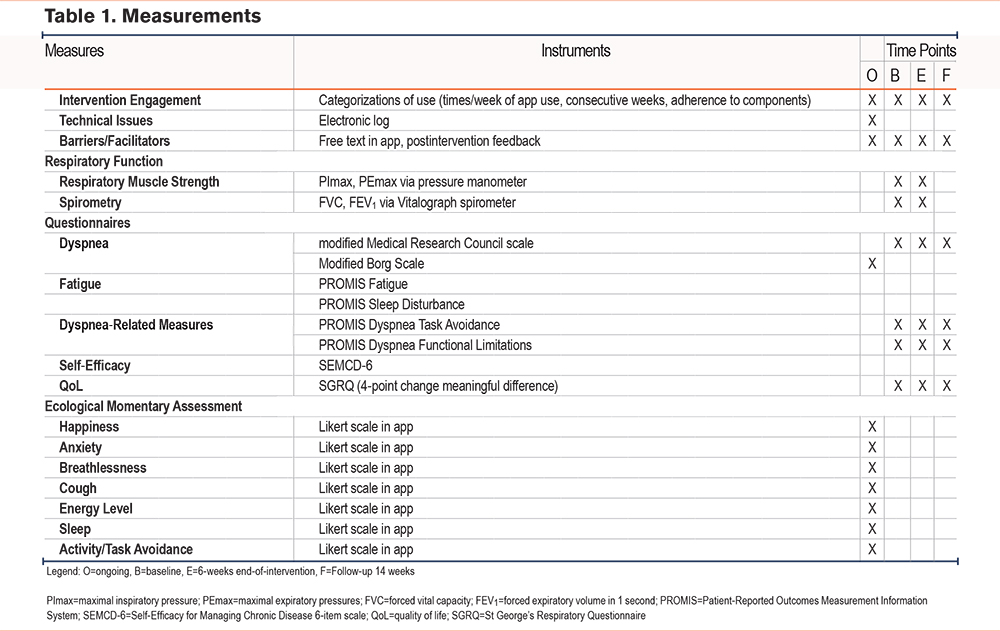
Following consent, participants were randomized 1:1 using a computer-generated probability stratified random sampling scheme into the control (n=15) or intervention (n=15) group. Both the principal investigator and the study biostatistician were blinded to study allocation. All measures were collected at baseline and 6 weeks following the intervention, with a follow-up phone call at 14 weeks from baseline (Table 1). Pulmonary function was assessed via spirometry (FVC and FEV1) measured 3 times on a computerized spirometer (Compact, Vitalograph; Buckingham, United Kingdom) in a pulmonary lab. All spirometry assessments were performed by a respiratory therapist unaffiliated with the study and blinded to group assignment and study procedures. The results of the best of 3 efforts were reported for spirometry and respiratory muscle strength. Respiratory muscle strength was assessed with a pressure transducer by measuring the PImax (cmH20) and the PEmax (cmH20) at residual volume and total lung capacity, respectively, with a mouthpiece that has a small air leak to prevent pressure generation by glottis closure.
Intervention Group
Respiratory muscle strength training devices were calibrated at 70% of individual baseline PImax. The intervention group performed RMST 5 days per week using a calibrated respiratory muscle strength trainer.25 The 6-week respiratory muscle strength training intervention was adapted from previous RMST training regimens32-34and comprised: (1) 5 training days per week using a combined IMST/EMST training device with Bluetooth-enabled frequency feedback to determine adherence and precise timing for device threshold intensification (i.e., increasing resistance training); (2) individualized, progress-based message training reminders and prompts; and (3) EMA function in the mobile app to monitor symptoms and track training sessions and adherence. Consistent with other muscle strength training programs, strength training exercises are conducted at regular intervals during the week (5 breaths, 5 times a day, 5 days a week). Participants received graphical illustration of RESP-FIT training instructions with information on training (see the online supplement). If they chose to opt into notifications on their smartphone, they were prompted and/or reinforced via text messaging.
Control Group
The control group did not undergo RMST but were given a simplified version of the app to log symptoms via EMA if desired.
Data Collection
Demographic data, respiratory function measures, and measures related to symptom experiences were collected from both groups at 3 time points (Table 1). Instruments to assess symptom domains included Patient-Reported Outcomes Measurement Information System (PROMIS)â measures relevant to COPD including pain, fatigue, depression, anxiety, sleep, and dyspnea.35-37 Other dyspnea measures (PROMIS Dyspnea Task Avoidance and PROMIS Dyspnea Functional Limitations) assessed self-reported impact of dyspnea on daily tasks and activities such as preparing meals, walking up steps, and decision or likelihood of not engaging in tasks due to breathing discomfort. Self-efficacy domains measured with the Self-Efficacy for Managing Chronic Disease 6-item scale included symptom control, role function, emotional function, and communication with physicians.37
EMA
In addition to pre/post measures, participants were asked to record symptoms in the mobile app in “real time” as they occurred. They recorded ratings of breathlessness, cough, energy levels, how their COPD affected sleep the previous night, anxiety/stress, activity engagement, and emotions using a 3-point Likert scale. Breathlessness (dyspnea) was measured with a modified Borg scale on a 100mm visual analogue scale, which was converted to a 3-point scale in analysis.38 Participants in the intervention group also reported difficulty of respiratory muscle strength training and their perception of respiratory muscle strength improvement using a 5-point Likert scale. Daily use of RESP-FIT was encouraged, and training sessions were noted in a mobile activity log (to track adherence). Data were captured for each participant’s interaction with the app and each training session.
Data Analysis
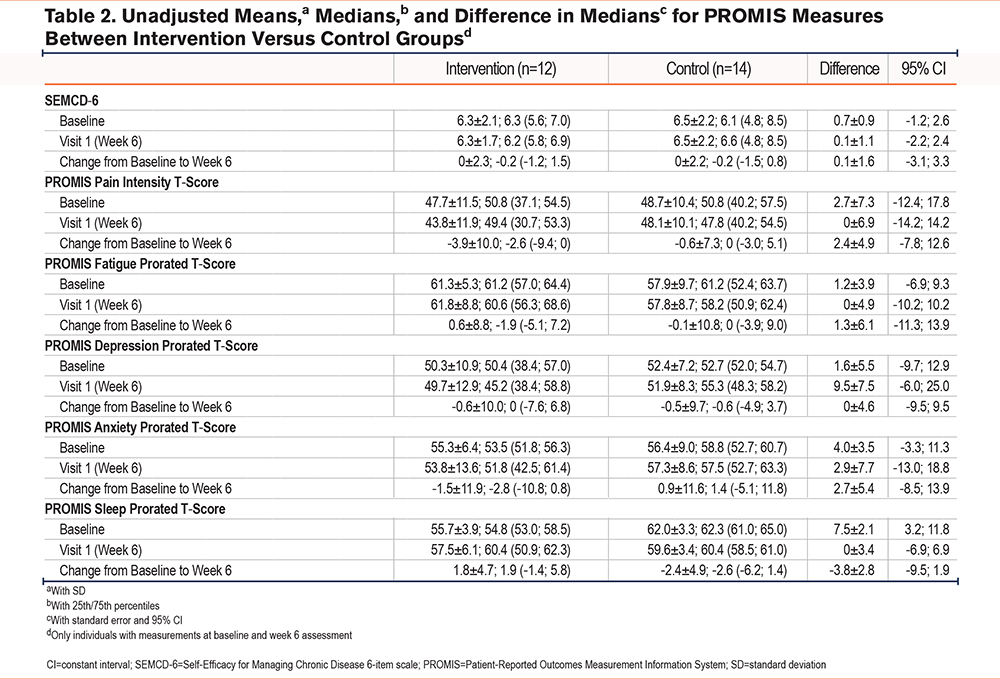
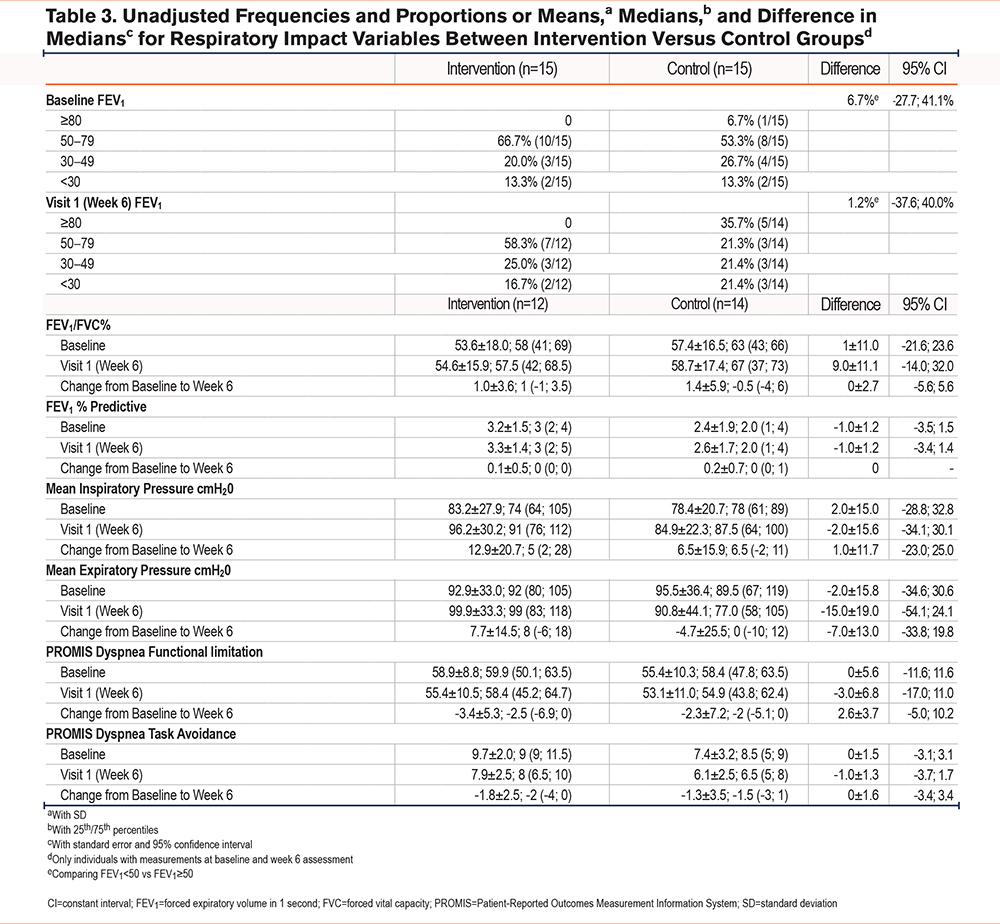
Secondary outcome measures, including dyspnea and fatigue, were analyzed as necessary inputs for the design of a future large randomized controlled trial (Tables 2-5). For the continuous outcome measures of self-efficacy, fatigue, and dyspnea, we used an intent-to-treat analyses and 95% confidence interval (CI) estimates of the within-group change scores (pre- to post-treatment) with precisions ranging from ±0.20 to ±0.78. These correspond to estimated standard deviations of change scores of these variables, ranging from 0.5 to 2.0. Study sample demographic and clinical characteristics were analyzed with descriptive statistics using frequencies, proportions, and measures of central tendency (means, medians) and variability (standard deviation [SD]) as appropriate. Average weekly scores of participant responses were collected for each question. Imputation methods were not utilized for missing data. Duplicates were identified and removed following review and consensus by the study statistician and principal investigator.
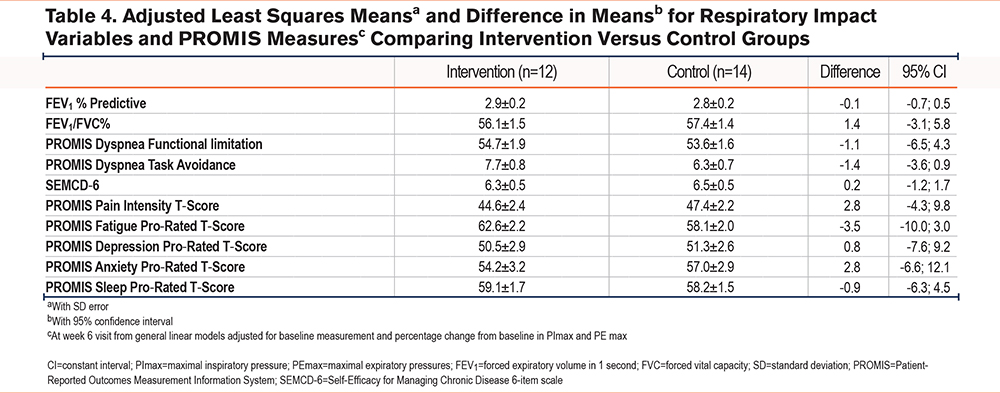
Unadjusted frequencies and proportions and difference in proportions with asymptotic standard error and 95% CI and means (with SD), medians (with 25th/75th percentiles) and difference in medians with standard errors and 95% CIs were calculated as appropriate for respiratory impact variables for the intervention (n=12) versus control (n=14) groups for all individuals with measurements at baseline and week 6 assessment. General linear models were used to obtain least squares means (with SD error) and difference in means with 95% CI for respiratory impact variables and PROMIS measures at the week 6 visit adjusted for baseline measurement and percentage change from baseline in maximal inspiratory pressure and maximal expiratory pressure to compare the intervention and control groups. All analyses used SAS Statistical Software Version 9.4 (SAS Institute Inc.; Cary, North Carolina).
Data Safety and Monitoring
This study employed the use of a Safety Monitoring Committee which convened semi-annually to review cumulatively reported and observed adverse events, monitor the study safety profile, and make recommendations regarding study modification, termination, and continuance.
Results
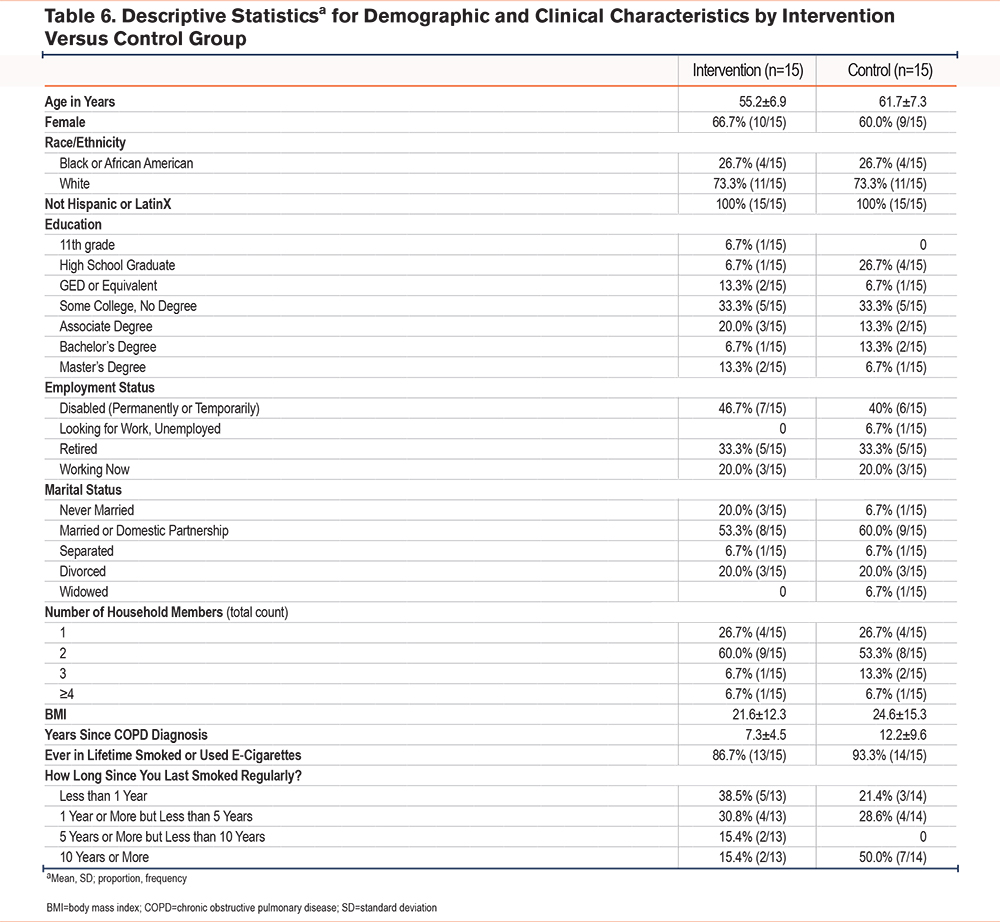
The majority of the participants (63.3%) were females who previously used cigarettes or e-cigarettes. Rural residents made up almost half of the study population (46.7%) and most participants (66.7%) lived in a medically underserved area. Demographic characteristics were similar between groups with a mean age of 55.2 years (SD=6.9) within the intervention group compared to 61.7 years (SD=7.3) within the control group. Additional demographic characteristics by group are presented in Table 6. There were 15 participants enrolled in each group, and a total of 12 participants in the intervention group and 14 in the control group completed the study (Figure 1, CONSORT diagram).
Symptom Domains
Pain intensity decreased slightly in the intervention group with a difference of -3.9 ± 10.0 compared to -0.6 ± 10.0 in the control group from baseline to 6-week follow up. Fatigue increased slightly in the intervention group with a difference of 0.6 ± 8.8 compared to -0.1 ± 10.8 in the control group from baseline to 6-week follow up. Depression decreased slightly in both the intervention (-0.6 ± 10.0) and control (-0.5 ± 9.7) groups, and anxiety decreased in the intervention group (-1.5 ± 11.9) but slightly increased (0.9 ± 11.6) in the control group. Sleep disturbance increased in the intervention group (1.8 ± 4.7) but decreased in the control group (-2.4 ± 4.9). Self-efficacy was mostly unchanged in both the intervention (difference of 0 ± 2.3 from baseline to 6-week follow-up) and the control group (difference of 0 ± 2.2 from baseline to 6-week follow-up), with a 0.1 difference between the 2 groups. The intervention group reported improvement in reported dyspnea functional limitations (-3.4 ± 5.3) compared to the control group (-2.3 ± 7.2). Finally, dyspnea task avoidance improved in the intervention group (-1.8 ± 2.5) and the control group (-1.3 ± 3.5). Results from questionnaires at baseline and 6-week follow-up are displayed in Table 2.
Pulmonary Function
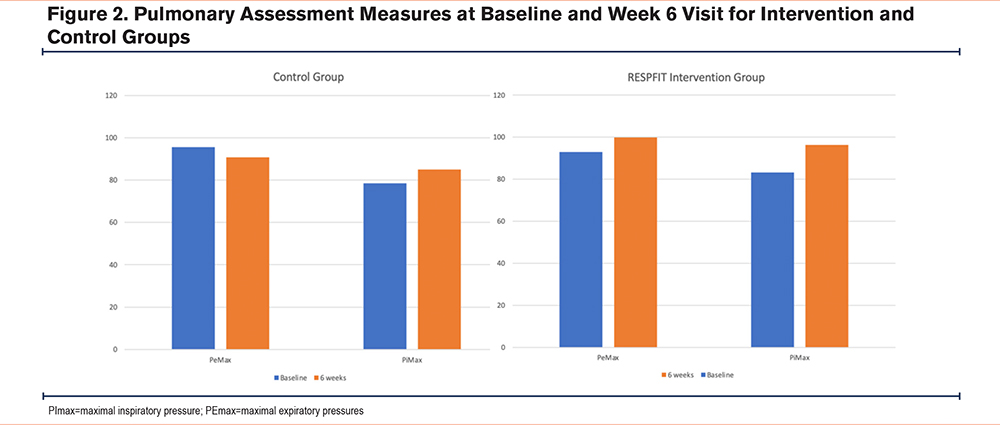
Postintervention spirometry was similar between groups, however, those within the intervention group demonstrated a small, 1% improvement in FEV1/FVC and the control group a 1.4% improvement in FEV1/FVC. In the intervention group, who completed 6 weeks of respiratory muscle strength training, PImax increased 12.9cmH2O and PEmax increased 7.7cmH2O from baseline to 6-week follow-up (Figure 2). In the control group, PImax increased 6.5cmH2O and PEmax decreased 4.7cmH2O from baseline to 6-week follow-up (Figure 2). Results from pulmonary assessment measures at baseline and 6-week follow-up are displayed in Tables 3 and 4 and Figure 2.
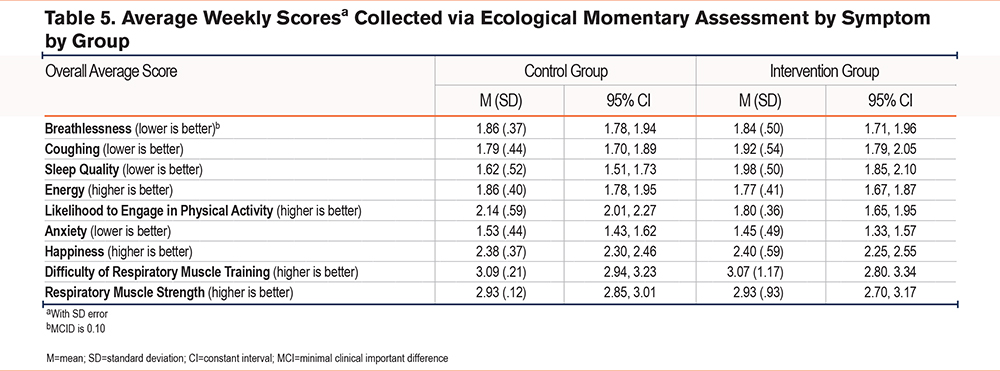
EMA Symptom Experience
Through the intervention period, participants reported symptoms in real-time (as they occurred) via a total of 14,388 data points via EMA in the mobile app. Overall, participants in the intervention group reported improved symptoms of breathlessness with a mean score of 1.84 (SD±.50) compared to 1.86 (SD±.37) in the control group, with minimal clinical important difference (MCID) being 0.10 (see Table 5). The intervention group also reported lower levels of anxiety with a weekly mean score of 1.45 (SD±.49) compared to 1.53 (SD±.44) in the control group. Finally, the intervention group reported higher levels of happiness compared to the control group with a weekly mean score of 2.40 (SD±.59) versus 2.38 (SD±.37) respectively. Participants in the control group reported less coughing with a mean weekly score of 1.79 (SD±.44) compared to 1.92 (SD±.54) in the intervention group. Reduced sleep quality and less energy were also reported by the intervention group, 1.92 (SD±.54) and 1.77 (SD±.41) respectively, compared to the control group (1.62 [SD±.52] and 1.86 [SD±.40]). EMA symptom reports are in Table 5.
Discussion
Results from this study provide insight into the effects of a technology-enhanced combined inspiratory and expiratory muscle strength training program. While this was a pilot and feasibility study not intended to determine intervention efficacy, findings indicated potential effects to generate sound hypotheses and support further testing of the intervention in an adequately powered efficacy study.
Symptom Domains and Experience
Overall, participants in the intervention group reported improved symptoms of breathlessness, lower levels of anxiety, and higher levels of happiness via EMA. However, unexpectedly, participants in the intervention group also reported reduced sleep quality and less energy compared to the control group. As a result of these findings, we hypothesize that a combined RMST program will improve emotional state and symptoms of breathlessness in COPD, but may contribute to fatigue and sleep disturbance, at least in the short-term. Additional work is needed to clarify the effects of RESP-FIT on emotions, anxiety, and levels of happiness to elucidate potential mechanisms of effect. These findings support the need for more robust operationalization of these concepts both as baseline and postintervention measures, and measurements over longer time periods via EMA.
It is possible that the introduction of a new exercise training regimen, in the form of RMST, contributed to the unexpected finding of increased fatigue. Muscle fatigue following training is also a possible explanation for reduced sleep quality (in terms of more sleep disturbances) and less energy (reported via EMA) reported in the intervention group. A possible solution is that training at 70% max could be reduced (to 50%–60%) to mitigate fatiguing effects. The concept of fatigue must be further operationalized in future studies to distinguish between muscular fatigue (related to exercising of the respiratory muscles), dyspnea- or COPD-related fatigue, or another unidentified contributor to fatigue. Additionally, adding additional pre-intervention EMA measures followed by evaluation of symptoms over extended time periods can assess for potential physiologic adaptation to training.
A score of 50 on the PROMIS measures is the average for the U.S. general population with an SD of 10 (Table 4). While this is reflected in our study population, it is possible that individual COPD status may have been a confounding variable on some of the subjective measures. COPD is a highly variable disease associated with periods of exacerbation, which are associated with burdensome symptoms. The high confidence intervals were wide, indicating that our sample was reporting on very different symptom experiences which may have affected their symptom ratings. This supports the need for a large-scale investigation with participants stratified by disease severity, rurality and geographic region (to explore environmental triggers), and exacerbation status to explore these relationships. Additionally, further work should incorporate a qualitative approach for a more robust understanding of phenomena. Participants in the control group still rated their symptoms and activity using the mobile app, so it is a possibility that there were effects in some subjective outcomes from the app itself being a confounding variable. Regular self-assessment and rating of disease status and symptoms over a 6-week period may affect individual processing of individual experiences in some unintended way. Thus, a “usual care” control group with no EMA measurement should be added to future studies.
Spirometry and Physiological Measures
As expected, spirometry remained unchanged in both the intervention and control groups. RMST is not expected to change airflow obstruction severity, but rather to improve mechanical strength and pressure generation for airway clearance and breathing. Supporting our hypothesis of improved respiratory muscle strength, mean inspiratory pressure (+12.9cmH2O) and mean expiratory pressure (+7.7cmH2O) improved in the intervention group from baseline to 6-week follow-up. In the control group, mean inspiratory pressure improved (+6.5cmH2O), but mean expiratory pressure decreased (-4.7cmH2O). While we expected PImax to increase in the intervention group, it is unknown why PImax improved in the control group. It is possible that it was a task learning effect, or situational variations (i.e., if someone had a cold or was unknowingly beginning an exacerbation period during baseline measures) or increased activity (if the mHealth component acted as a confounding variable) may have contributed to this increase, or some other unknown confounding variable that we were unable to identify. Some participants in both groups had close to normal baseline PImax levels, which may limit the potential improvement and subsequent clinical impact from RMST. Future studies should specifically investigate this with a focus on participants with respiratory muscle weakness.
EMA Measures
Dyspnea decreased in the intervention group, indicative of MCID for dyspnea (1 unit on a modified Borg scale, or 10mm on a 100mm visual analogue scale). This is consistent with other RMST studies in healthy adults.39 While we evaluated changes in dyspnea over 6 weeks from participants in each group, future work is needed to investigate individual variations in dyspnea and conduct within-participant comparisons using EMA data. The intervention group also reported higher happiness and lower anxiety compared to the control group, supporting the generation of our hypothesis, which will be explored in a subsequent large-scale clinical trial. Activity level during EMA should also be considered during future studies.
With continually increasing physical wearable devices and technology-based interventions, new measures are needed to capture outcomes and behavioral changes.40 EMA is a tool to capture information clinically, so health care providers can identify trends, monitor severity of symptoms during individual events and over time, and investigate potential predictors of COPD exacerbations.41 Adding tracking of environmental factors such as airway pollutants or seasonal pollens along with self-reported EMA symptom data will provide valuable insight into the effect of environmental influences on COPD progression and exacerbations.
Intervention Accessibility
Our generated hypotheses, that the RESP-FIT intervention may improve COPD self-management and quality of life by increasing respiratory muscle strength and improving symptoms, supports the potential of RESP-FIT to offer future value and benefit to the COPD population. Notably, there were no differences in the engagement of patient populations from rural and medically underserved areas, supporting that RESP-FIT may be an accessible intervention regardless of geographic location or available resources. The burden and prevalence of COPD is high in rural and medically underserved areas, where patients face unique barriers and needs. In South Carolina, where the study was conducted, approximately one-sixth (17.6%) of residents live in poverty and many more face significant barriers to respiratory health care. Further, there is a shortage of specialized health care resources for patients with COPD in South Carolina, with 95% of South Carolina residents living in a Primary Care Health Professional Shortage area,42 and only 72 pulmonologists practicing in the state (or approximately 1 per 73,000 people). There is a strong need for remote, accessible COPD interventions that can be delivered in rural and medically underserved areas, and it is possible that RESP-FIT can address this need. Future work must investigate socioenvironmental factors with intervention accessibility, engagement, and outcomes.
Strengths and Limitations
To our knowledge, this is the first study to evaluate a technologically-enhanced combined-threshold inspiratory and expiratory muscle strength training program for individuals with COPD and explore effects on symptoms, PROs, and respiratory muscle strength. One strength of this study is that preliminary data were sufficient to generate hypotheses that will be used in future large-scale clinical trials. Another strength of our study is the engagement and representation of patient populations from rural and medically underserved areas, where the burden and prevalence of COPD is high.
There were some limitations to this study. Our sample was well educated, with 70% having earned a college degree or having completed some level of postsecondary education, which limits generalizability to those with lower educational and literacy levels. Comorbid conditions contributing to dyspnea may have been confounding with the inclusion of moderate-to-severe COPD by FEV1/FVC. Technology may also be a potential limitation for some patients. While the overall use was high, it is possible that issues with technology (Bluetooth connections and mobile app freezing) may have influenced the engagement with the intervention. All participants had mobile or cellular data access, and this may have excluded individuals without these resources. Finally, it is unknown how digital literacy may have contributed to intervention engagement and this may be an unknown barrier.
Future Research
While promising, this work was focused on exploration of intervention effects for hypothesis generation. Thus, a large-scale clinical trial is needed to determine efficacy in a population of individuals with COPD. Future studies should include a usual care control group for comparison, stratify by disease severity, and evaluate potential effectiveness on exacerbations. Future studies should also investigate data collection strategies that utilize EMA to inform development of a validated, standard language for EMA measurement of dyspnea in specific patient populations to allow for more accurate measurement of dyspnea in real-time. We used a standard Borg scale which has been validated for MCID, but it is possible that another measurement tool such as the mMRC may be valid for EMA of dyspnea. Studies should investigate RESP-FIT across various pathological conditions to better inform patient interventions and clinical management of patients with respiratory diseases and dyspnea. Finally, as respiratory health inequity is a determinant of overall respiratory health, social determinants of health must be considered in regard to intervention accessibility, engagement, and outcomes in future studies.
Conclusion
A remote, mHealth-enhanced 6-week respiratory muscle strength training intervention, RESP-FIT, supports improved respiratory muscle strength (via maximum inspiratory pressure generation) and COPD-related symptoms but may contribute to muscular fatigue. This study was sufficient to generate sound hypotheses that will be tested in a future large-scale clinical trial to determine efficacy of RESP-FIT in adults with COPD.
Acknowledgements
Author contributions: SNM, MM, MN, RJTII, DML, CS, MM, MCP, TJK, and PWD all made substantial contributions to the conception and design of the study, the acquisition of data, data analysis and interpretations, and the drafting and/or revision of the manuscript. Each author contributed significantly to the intellectual content of the article, critically reviewed the work, and provided final approval of the version to be published.
Data sharing statement: As a condition of this National Institutes of Nursing Research (NINR) award, de-identified patient data will be shared by the researchers with the NINR and stored electronically on an NIH password protected secure server (https://cdrns.nih.gov/). The purpose of sharing this information is to build a NINR repository of data using common data elements for future research purposes among the general scientific community and for public health benefit. Please see ClinicalTrials.gov ID: NCT03652662 for a detailed data sharing report.
Other acknowledgments: Authors would like to acknowledge the editorial contributions of Susan McCabe, MSHI, ANP, CNE.
Declaration of Interests
The authors declare there are no conflicts of interest that may have inappropriately influenced the work presented in this manuscript.