Running Head: Improved Alpha-1 Antitrypsin Deficiency Screening
Funding Support: This quality improvement initiative was supported by an educational grant from Takeda Pharmaceuticals U.S.A., Inc. The funders did not have a role in the quality improvement initiative design, data analysis, data interpretation, or writing of the report.
Date of Acceptance: September 25, 2024 | Publication Online Date: October 3, 2024
Abbreviations: AAT=alpha-1 antitrypsin; AATD=alpha-1 antitrypsin deficiency; COPD=chronic obstructive pulmonary disease; EBNHC=East Boston Neighborhood Health Center; EHR=electronic health record; LCL=lower control limit; PFT=pulmonary function test; UCL=upper control limit; URGs=underrepresented racial or ethnic groups
Citation: Wilson AA, Bora C, Silva C, et al. A multimodal intervention to improve guideline-based screening for alpha-1 antitrypsin deficiency in a community health setting. Chronic Obstr Pulm Dis. 2024; 11(6): 582-590. doi: http://doi.org/10.15326/jcopdf.2024.0540
Online Supplemental Material: Read Online Supplemental Material (436KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an inherited cause of lung and liver disease resulting from mutations in the SERPINA1 gene that promote the misfolding and reduced secretion of alpha-1 antitrypsin (AAT) protein into the blood. The accumulation of misfolded protein aggregates within hepatocytes causes fibrotic liver disease in some affected patients. In addition, low circulating levels of AAT and the associated protease/antiprotease imbalance in the lungs make affected individuals susceptible to developing chronic obstructive pulmonary disease (COPD)/emphysema.1 Although numerous variants in SERPINA1 exist in the population, the “Z” variant is responsible for most clinically significant disease. Individuals who inherit 2 mutant alleles (“ZZ” genotype) have a high risk of developing lung and liver disease, whereas those who inherit a single mutant allele (“MZ” genotype) have a moderate risk of liver disease2,3and exhibit an increased risk of COPD only in the context of an additional injury, such as cigarette smoking.4 AATD is estimated to account for approximately 2% of all cases of COPD in the United States, supporting guidelines that recommend screening for AATD of all individuals diagnosed with COPD regardless of age or ethnicity.5-10
The early identification of AATD is crucial to facilitate lifestyle changes and timely treatment with AAT augmentation therapy, a specific treatment for AATD that can slow the progression of lung damage and prolong the time to lung transplantation or death.11-13 Despite the existence of screening guideline recommendations, it is estimated that up to 95% of patients with COPD are never tested for AATD.8-10,14 A variety of barriers to screening have been identified, including limited awareness of AATD, uncertainty regarding when to suspect the disease, and lack of knowledge about appropriate diagnostic tests and available treatments.15 These barriers are potentially compounded in individuals from underrepresented racial or ethnic groups (URGs) because AAT mutant gene frequencies are less well established in these populations and clinical trials testing the efficacy of augmentation therapy have largely been restricted to individuals of European descent.12,16,17
In this project, we implemented a quality improvement initiative for primary care clinicians at a large, racially and ethnically diverse community health center to improve their identification of patients who meet guideline-based criteria for AATD screening and optimize their processes for laboratory testing to diagnose AATD.
Methods
Project Setting and Participants
The cohort for this quality improvement initiative included primary care and advanced practice providers from the East Boston Neighborhood Health Center (EBNHC), which includes the following clinic locations: South End Community Health Center, Winthrop Neighborhood Health, PACE Lewis Mall, PACE Revere, and PACE Winthrop. EBNHC is the largest community health center in Massachusetts and one of the largest in the nation, serving nearly 82,000 patients and employing more than 1300 staff members.18 This project was approved by EBNHC’s board in June 2022.
Quality Improvement Criteria
The Boston Medical Center Institutional Review Board determined this work was consistent with the definition of a quality improvement project and was not considered research. As such, the initiative was exempt from the board’s review. This determination occurred prior to the onset of the quality improvement intervention.
Interventions
Educational Sessions
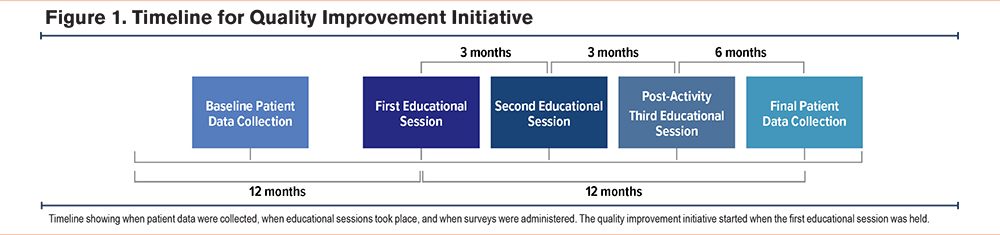
Clinicians were invited to participate in a series of 3 live educational sessions, held every 3 months between July 2022 and January 2023 via virtual conferences (Supplementary Table S1 in the online supplement). Each educational session consisted of a 1-hour grand rounds session that included information on the genetic attributes and disease manifestations of AATD, guideline recommendations for AATD screening and diagnostic testing in patients with COPD, case studies, and the role of augmentation therapy in the treatment of patients with COPD and AATD. Guidance was also provided on using the electronic health record (EHR) software, Epic, to order testing, interpret test results, and make referrals for specialty care for those diagnosed with AATD.
EHR Software Modifications for AATD Testing and Referral
The EHR software, Epic, at EBNHC and the 5 additional clinic locations was modified to alert clinicians in the health maintenance summary if a patient with a diagnosis of COPD or emphysema was overdue for one-time AATD screening (Supplementary Figure S1 in the online supplement). The alert directed clinicians to the Care Gap SmartSet to order a prepopulated AAT total test to measure AAT levels (Supplementary Figure S2 in the online supplement). The normal reference range for AAT was 83 to 199mg/dL as determined by the commercial laboratory system Quest Diagnostics. Results of less than 83mg/dL were considered deficient and necessitated additional testing to confirm an AATD diagnosis. If a patient’s AAT level was below 83mg/dL, a reflex AAT phenotype test would automatically be triggered and a written report would be generated, indicating whether the patient had 1 or 2 deficient alleles (Supplementary Figures S3 and S4 in the online supplement). Patients with 1 or 2 abnormal alleles could then be referred for specialty care at a local tertiary care facility by inputting a specific referral within Epic (Supplementary Figure S5). Additional information was provided in a decision support tool available within the EHR reference guide that included rationale for testing, instructions for ordering the blood test, guidance on interpreting results, specific patient counseling topics, and a review of the referral process.
In addition to the Care Gap SmartSet orders, a SmartPhrase was created to mitigate barriers that clinicians may experience when ordering AATD testing. The SmartPhrase could be populated into a clinical note using a dotphrase “.alpha1”; additionally, the SmartPhrase automatically linked any prior AAT total test result and outlined eligibility and frequency for AATD testing. It provided specific language for patient counseling on the rationale for testing and the implications of different possible test results. Explicit instructions were also provided on how to interpret AATD test results, with the goal of increasing clinicians’ confidence in their ability to understand the results and convey results to patients. Lastly, guidance was given on identifying patients who should be referred for treatment or familial genetic counseling. The Care Gap SmartSet was also loaded into the EHR sidebar clinical reference guides.
Survey Development and Administration
Online surveys were used to gather data regarding clinician knowledge of and practices related to AATD screening before and after the 3 educational sessions were held. Baseline and postactivity survey questions were developed using input from faculty experts and needs assessment research. Surveys were administered online, and links were emailed to clinicians practicing at EBNHC. A small stipend was offered to those who completed the surveys. Survey respondents included physicians and advanced practice providers. Baseline survey data were collected prior to the first educational session. Postactivity survey data were collected just before the third educational session (Figure 1). Results were analyzed descriptively.
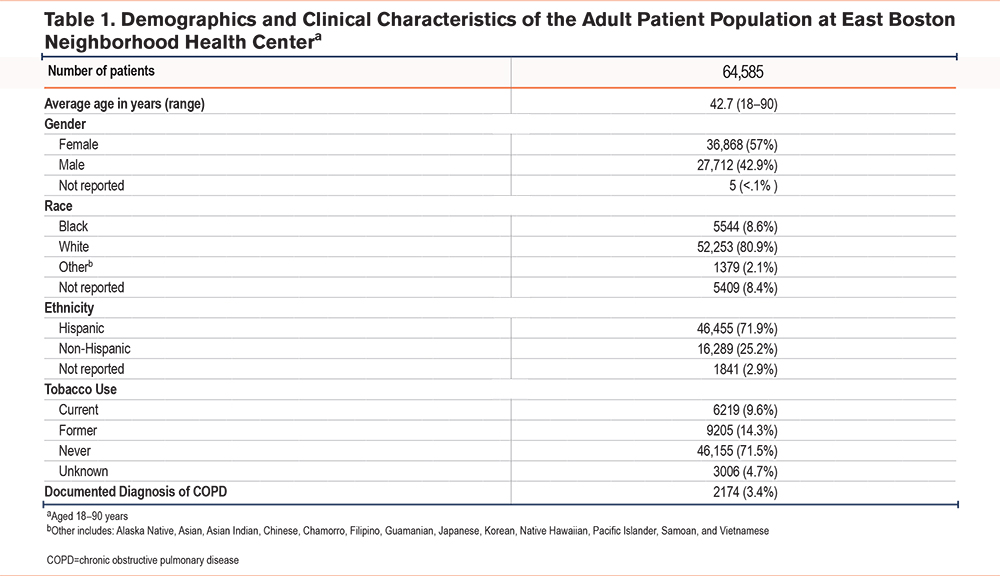
Patient Data Collection and Analysis
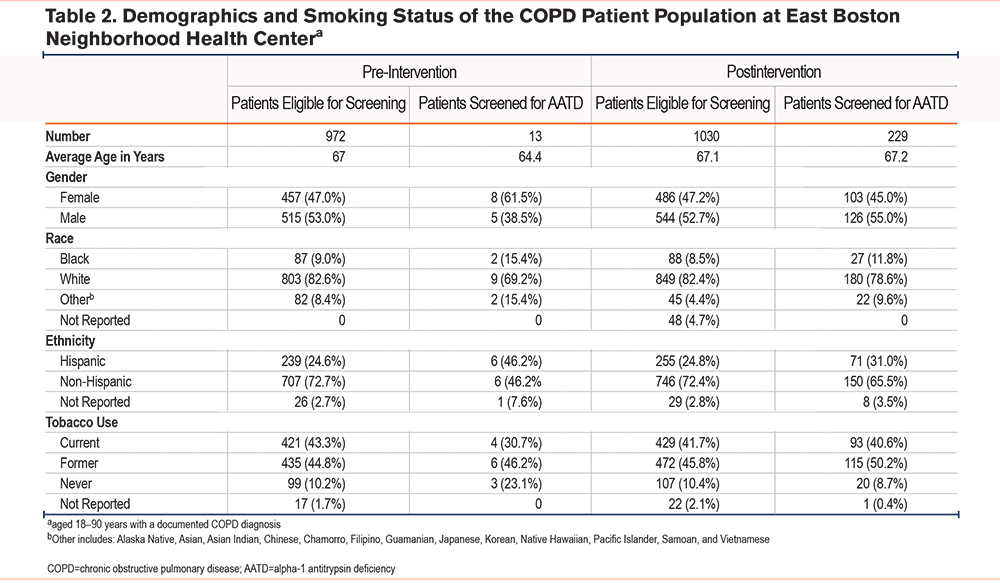
Deidentified patient data were collected from the EHR of EBNHC, South End Community Health Center, Winthrop Neighborhood Health, PACE Lewis Mall, PACE Revere, and PACE Winthrop. The demographics and clinical characteristics of the total patient population seen at the 6 locations are shown in Table 1. Patients were included in the COPD patient population if they were actively in care (defined as having a primary care visit in the past year), aged between 18 and 90 years, and had a diagnosis of COPD or emphysema. Patients were excluded if they had no evidence of a COPD or emphysema diagnosis, were younger than 18 years, or were older than 90 years (Table 2). Among those receiving care at the 6 clinic locations, a total of 972 patients had a COPD/emphysema diagnosis, defined as an International Classification of Diseases-Tenth Revision code and/or Epic list diagnosis, and were seen for at least 1 primary care visit in the 12 months prior to the intervention. A total of 1030 patients who met these criteria were seen in the 12 months after the start of the intervention. Baseline patient data collected consisted of the number of patients with an EHR-documented AATD test result ordered in the 12 months prior to the start of the initiative or first educational session (from July 2021 to June 2022). Postactivity patient data were collected in the 12 months after the start of the initiative or first educational session (from July 2022 to July 2023). Both sets of data included patients who met the inclusion criteria and had a new AATD test result documented during those respective time frames (Figure 1). The baseline and postactivity data were analyzed using a paired T-test for significance with Microsoft Excel software and a C-chart with QI Macros, Microsoft Excel.
Results
Baseline Alpha-1 Antitrypsin Deficiency Testing: Reported Versus Actual Results
In the baseline survey, 58% of respondents (n=36) indicated they never screen patients with COPD for AATD and 36% reported they sometimes screen patients with COPD for AATD. Only 8% of respondents reported having seen a patient with AATD in their practice. These baseline survey questions are included in Supplementary Table S2 in the online supplement.
Among 972 patients with COPD seen in the 12-month period preceding the start of the initiative (July 2021 to June 2022), 13 patients (1.3%) underwent AATD screening (Table 2). An additional 66 patients already had an EHR-documented result from AATD screening that had been ordered prior to the baseline data collection (1999 to June 2021). Survey respondents reported the following barriers to AATD screening: lack of knowledge about the link between COPD and AATD, limited training and experience regarding AATD screening, the absence of screening from their usual practice, uncertainty regarding ordering tests and interpreting results, and uncertainty about where to refer patients with an abnormal test result.
Postactivity Alpha-1 Antitrypsin Deficiency Testing: Reported Versus Actual Results
In the postactivity survey, 48% of respondents (n=23) indicated that they had ordered a test for AATD in the 6-month period that followed the first educational session, 39% of respondents had not ordered an AATD test, and an additional 13% of respondents indicated not having seen a patient with COPD who qualified for guideline-based screening. Survey respondents reported that low/deficient levels of AAT were not detected in any patients tested, and no patient referrals for AATD specialty care occurred in the 6 months following the intervention.
When asked what part of this education was most useful to their practices, participating clinicians cited the entire quality improvement initiative, including the Care Gap SmartSet orders and SmartPhrase that were added to the EHR software, as well as the provided education about clinical practice guidelines for AATD screening. Additionally, participating clinicians stated that they were previously unaware of the practice guidelines and the need to screen individuals with COPD.
Postactivity survey results identified ongoing challenges, including limited confidence when explaining the implications of the test results to their patients, lack of comfort reviewing the need for AATD screening with other providers’ patients being seen for acute problems due to COVID-19 or as a result of staffing disruptions, and the concern that patients may be first misdiagnosed with asthma instead of COPD. Lack of time was also identified as a barrier to AATD screening. The postactivity survey questions are included in Supplementary Table S3 in the online supplement.
An increase in AATD screening rates was reflected in the patient data obtained from the EHR capturing the 12-month period following the start of the initiative. Overall, among EBNHC’s 6 clinic locations, 229 (22.2%) of the 1030 patients with COPD were screened for AATD in the 12 months following the first educational session (Table 2), a significant increase (P<0.001) in AATD screening rates in the COPD population of EBNHC relative to the pre-intervention period (Figure 2). AAT levels below 83mg/dL triggered phenotype testing for 9 patients. Of those, 1 sample was found to be heterozygous for the rare F allele (phenotype PI*MF), and no cases of severe AATD were identified.
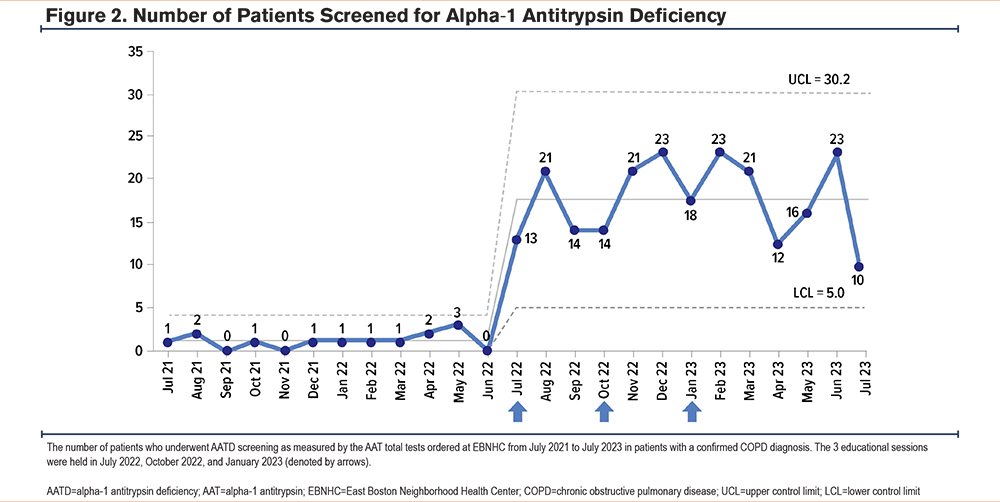
Increases in the number of eligible patients screened for AATD were also observed after each educational session. After the first educational session, 13 patients were screened in July 2022 and 21 patients were screened in August 2022. Similar trends were observed after the second educational session, held in October 2022, with 21 and 23 patients undergoing screening in November 2022 and December 2022, respectively. After the third and final educational session, held in January 2023, 23 patients were screened in February 2023 (Figure 2).
Discussion
Summary of Findings
Our project demonstrates that the implementation of a quality improvement initiative consisting of EHR notifications (Care Gap alerts and supportive information posted as a SmartSet in the Epic EHR software) and educational sessions (live sessions held over Zoom) increase AATD screening rates among primary care and advanced practice providers in a large community health clinic setting.
A variety of studies have previously applied either EHR-based or educational interventions to increase AATD testing rates. Rahaghi et al19 found that adding a physician alert to pulmonary function test (PFT) reports identifying moderate COPD (Global initiative for chronic Obstructive Lung Disease [GOLD]9 stage 2) or greater airflow obstruction increased AATD screening rates from 6% to 13% over the course of 6 months. Similarly, Jain et al20 found that adding a guideline-based alert to the EHR prompting AATD testing of patients with airflow obstruction based on PFTs resulted in screening rates increasing from 4.7% to 15.1%. Targeting patients rather than clinicians, Lam et al21 found that using the EHR to send electronic patient messages offering free, home-based AATD testing to patients prescribed inhalers for COPD resulted in a testing rate of 3.8% (baseline testing prior to the intervention was not reported). Alternatively, respiratory therapist participation in an online educational program focusing on AATD was associated with referral of patients for AATD testing.22 Two recent studies tested individual elements included in this initiative: an EHR-based clinical decision tool was evaluated in a Veterans Affairs population, and an educational intervention was studied in an academic pulmonary practice, both leading to increased screening of patients at risk of AATD.23,24
Our initiative is distinguished by its community health setting and by its combination of interventions that included: (1) EHR notifications reminding clinicians to screen patients with COPD for AATD, and (2) live, interactive educational sessions addressing AATD and associated screening guidelines, diagnostic testing, available treatments, and patient resources.
Promotion of Health Equity
Individuals from URGs and underserved populations have been underrepresented in the AATD literature and absent from AATD clinical drug trials.12,17 Implementing this initiative in a community health center where 81.38% of the nearly 82,000 total patients represent racial and ethnic minorities18 was an opportunity to ensure that this population receives guideline-based testing and is included in the literature defining AATD rates, which is an essential step if such patients are to be identified for participation in future clinical trials.25
Implications for Practice
The combination of educational sessions and EHR interventions was beneficial in helping increase AATD screening rates at a large community health clinic. To maximize participation, 3 educational sessions were offered during a scheduled grand rounds time as accredited programs for which participating clinicians could choose to receive continuing medical education credit. To demonstrate the need for increases in rates of AATD screening, baseline survey results and EHR findings showing that only 1.3% of patients with COPD seen at the 6 clinic locations in the prior 12 months had been tested for AATD were shared with participating clinicians. Educational sessions consisted of short didactic presentations to increase knowledge of AATD, including the associated screening guidelines, diagnostic testing approach, and treatment. Information was provided on the updates made to the Epic EHR software. Participating clinicians also had opportunities to ask questions and discuss barriers to AATD testing.
Changes to the clinicians’ existing workflow processes were developed to facilitate practice changes. A Care Gap SmartSet was added to the Epic EHR software to alert clinicians if a patient with COPD or emphysema had not been screened for AATD, prompting them to order an AAT test. A reflex phenotype test was automatically triggered for patients with deficient AAT levels to confirm the AATD diagnosis, identify AAT protein variants, and avoid the need for follow-up visits and blood draws. Additional information was provided in a best practice alert guide in the Epic EHR software on the rationale for testing, interpretation of results, specific patient counseling, and facilitation of a referral, which could be accessed using an Epic SmartPhrase. These EHR interventions were intended to overcome barriers that clinicians experience when screening a patient for AATD, as well as facilitate the interpretation of test results and coordinate referrals. Finally, these EHR interventions were designed to help sustain practice changes after the end of the quality improvement initiative, especially as staffing changes and guidelines evolve.
Few health center resources were required to implement this broad intervention across 6 clinical sites. The health center’s information technology team required minimal hours to make necessary modifications to the Epic EHR system. The laboratory team worked with the billing department to ensure AATD tests would be coded and billed correctly in the EHR. The Epic tools were codeveloped by representatives from the health center and faculty, using both health center clinician knowledge and faculty expertise to build tools that were tailored to the specific needs of the health center. Overall, the implementation of Epic tools required minimal resources and could be easily reproduced in other community health centers.
While testing rates varied over time during the intervention period, we noticed increases following each educational session, with some declines between sessions. We speculate that time was necessary for clinicians to become familiar with the testing approach and to fully incorporate it into their practice, consistent with the gradual uptake pattern observed in the incorporation of other evidence-based practices.26 It is possible that reinforcement, an important part of quality improvement, will be important to maintain high testing levels over time.27 Importantly, the Care Gap reminder included in our Epic intervention notifies clinicians of the need to test eligible patients and serves this purpose.
Despite the improvements in AATD screening rates seen in our quality improvement initiative, barriers to AATD screening remain among participating clinicians. In the postactivity survey, respondents noted continued challenges articulating to patients the rationale for screening, explaining test results, and finding sufficient time to implement screening during already busy clinic visits. Implementing tools to facilitate AATD screening is key to addressing many such barriers, but time limitations for clinicians may continue to be a challenge, even for a well-supported practice change. This intervention increased clinician time dedicated to patient education and test result review. However, clinicians appeared to be willing to take on extra work in their already busy schedules to ensure that patients would be offered AATD testing, as evidenced by the increased and sustained testing levels over time (Figure 2). Other barriers have previously been hypothesized to limit AATD diagnostic testing, including barriers to changing physician behavior.28 To continue to improve AATD testing rates in the future at our community health center, we plan to: (1) emphasize formal diagnostic testing for COPD and asthma in continuing medical education presentations, and (2) reinforce our prior continuing medical education teaching on the rationale for and logistics of screening for AATD.
Some limitations were encountered in the implementation of our quality improvement initiative. For example, clinician availability prevented many from attending the 3 educational sessions. Additionally, the follow-up period for our project was limited to 12 months from the first educational session, which was not adequate to capture all return visits of patients with COPD. The COVID-19 pandemic contributed to fewer patients presenting to the clinic for in-person care and more patients relying on telehealth visits, which prevented clinicians from being able to order an AATD test at the point of care. Patients were also lost to care during the COVID-19 pandemic and did not later return for follow-up visits. Lastly, AATD tests may not have been ordered if patients presented to the clinic with COVID-19 symptoms or other acute illnesses.
Conclusions
A quality improvement initiative including educational sessions and EHR modifications was successful in increasing guideline-driven diagnostic screening rates for AATD in patients diagnosed with COPD among primary care clinicians at a large community health center. Additional evaluation beyond 12 months of follow-up is needed to determine the long-term impact of this quality improvement initiative.
Acknowledgements
Author contributions: AAW, JLW, NS, JS, CQ, and DG were involved in the conceptualization of the manuscript. AAW, CB, CS, JLW, JS, CQ, and DG were involved in the methodology of this study. AAW, CB, JLW, and NS performed the data curation and AAW, CB, CS, NS, and DG performed the data analysis. JS wrote the original draft of the manuscript, and AAW, CB, CS, JLW, NS, JS, CQ, and DG reviewed and edited. CB, CS, JLW, NS, JS, CQ, and DG were involved in project administration. All authors reviewed and approved the manuscript prior to submission.
The authors thank Rebecca L. Julian, MS, ELS, Med-IQ, and Laura Rafferty, ELS, Med-IQ, for editorial assistance, Yvonne Walker, Med-IQ, for graphics assistance, Charles Williams, MD, Boston HealthNet at Boston Medical Center, for statistical analysis, and Sam Choi, Epic, for Epic EHR support and data analytics.
Declaration of Interests
AAW has received grant research support from Grifols and Beam Therapeutics. The remaining authors have no conflicts of interest to declare.