Running Head: Effect of Sex on COPD Triple Therapy Outcomes
Funding Support: This work was funded by GSK (study number CTT116855; NCT02164513). The funders of the study had a role in the study design, data analysis, data interpretation and writing of the report.
Date of Acceptance: October 31, 2024 | Publication Online Date: November 6, 2024
Abbreviations: AEs=adverse events; AESIs=adverse events of special interest; BMI=body mass index; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FF=fluticasone furoate; ICS=inhaled corticosteroid; IMPACT=InforMing the Pathway of COPD Treatment; LAMA=long-acting muscarinic antagonist; LRTI=lower respiratory tract infection; LS=least squares; SAEs=serious adverse events; SD=standard deviation; SGRQ=St George's Respiratory Questionnaire; SITT=single-inhaler triple therapy; SMQ=standardized Medical Dictionary for Regulatory Activities queries; UMEC=umeclidinium; VI=vilanterol
Citation: Alberola AH, Nogal NB, Miranda AB, Lipson DA, Tombs L, Han MK. The effect of patient sex on treatment outcomes in COPD: a post hoc analysis of the IMPACT trial. Chronic Obstr Pulm Dis. 2024; 11(6): 591-603. doi: http://doi.org/10.15326/jcopdf.2024.0541
Introduction
Globally, chronic obstructive pulmonary disease (COPD) is estimated to affect 10.3% of the population,1 with 11.8% of males and 8.5% of females living with the disease.2 COPD is traditionally seen as a disease predominantly affecting males, with females often experiencing underdiagnosis and diagnostic delays,3-5 potentially contributing to suboptimal treatment and consequently poorer outcomes.4-7 In patients with COPD, both the physiology of the lung and pathophysiology of disease are reported to differ according to patient sex.8 Compared with males, females are reported to have more severe dyspnea9,10 and chronic cough,9 as well as a higher frequency of exacerbations,9,11-14 and more comorbidities including anxiety, depression,4,10,15 and osteoporosis.4,11 However, greater sputum production and more hospitalizations have been reported in males compared with females.11,16 Smoking is the main risk factor for developing COPD, and whilst a greater proportion of males reportedly use tobacco products, smoking in females has increased within high-income countries.17 In the United States and Europe, deaths from COPD among smokers have risen, with females having an equivalent risk of death as male smokers.18,19 Furthermore, the majority of nonsmoker patients with COPD are female, with exposure to biomass fuels reported to be a contributing factor.20,21 Despite these known differences between males and females, it is not known whether patient sex affects response to COPD inhaled treatment. An understanding of the impact of patient sex on outcomes may help predict treatment response and enable a more personalized approach to COPD treatment.
The InforMing the Pathway of COPD Treatment (IMPACT) trial demonstrated that single-inhaler triple therapy (SITT) with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) reduced exacerbations, improved lung function, reduced all-cause mortality, and improved health status in patients with COPD when compared with dual therapy (FF/VI or UMEC/VI).22,23 The effect of patient sex on the efficacy of triple versus dual therapy on clinical outcomes and safety has yet to be fully reported. Here, we aim to ascertain if patient sex affects treatment outcomes by evaluating the efficacy and safety of FF/UMEC/VI versus FF/VI and UMEC/VI in males and females in a post hoc analysis of the IMPACT trial.
Methods
Study Design
The IMPACT trial (GSK Study CTT116855/NCT02164513; N=10,355) was a phase 3, 52-week, randomized, double-blind, parallel-group, multicenter study that compared the efficacy and safety of once-daily SITT with FF/UMEC/VI 100/62.5/25μg with once-daily dual therapy with FF/VI 100/25μg or UMEC/VI 62.5/25μg, administered via the ELLIPTA dry-powder inhaler; the full study details have been published previously.22,24 Patients continued their existing COPD maintenance therapy during a 2-week run-in period after screening and prior to being randomized (2:2:1) to reflect routine clinical practice.
Study Population
Study eligibility criteria have been reported previously.22,24 Eligible patients were males and nonpregnant females, aged ≥40 years with symptomatic COPD (COPD Assessment Test [CAT] score ≥10) and with either a forced expiratory volume in 1 second (FEV1) <50% of predicted normal values and ≥1 moderate or severe exacerbation in the previous year, or an FEV1 50%–80% of predicted normal values and ≥2 moderate or ≥1 severe exacerbations in the previous year. All patients were required to have a ≥10 pack-year smoking history. Exclusion criteria included a current diagnosis of asthma; however, patients with a history of asthma were eligible to increase the generalizability of the trial. All patients provided written informed consent. The IMPACT trial was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval from local institutional review boards or independent ethics committees.
Endpoints
This analysis evaluated the following efficacy endpoints: (1) the annual rate of on-treatment moderate/severe exacerbations and risk (time-to-first) of on-treatment moderate/severe exacerbations; (2) change from baseline in trough FEV1 at Weeks 4, 28, and 52; and (3) mean St George’s Respiratory Questionnaire (SGRQ) total and domain scores and change from baseline in SGRQ total score at Week 52. Moderate exacerbations were defined as exacerbations requiring treatment with oral/systemic corticosteroids and/or antibiotics (not involving hospitalization or resulting in death). Severe exacerbations were defined as exacerbations that required hospitalization or resulted in death. Safety endpoints included exposure-adjusted incidence rates of adverse events (AEs), serious AEs (SAEs), and the number of AEs of special interest (AESIs).
All endpoints were stratified post hoc by patient sex (male/female). Further stratifications by age (<65 years, ≥65 years), baseline blood eosinophil count (<150 cells/µL, ≥150 cells/µL), and moderate/severe COPD exacerbation history (<2 moderate and no severe exacerbations, ≥2 moderate or ≥1 severe exacerbation; both assessed in the prior year) were also performed. Blood samples for eosinophil counts were taken at screening and Weeks 16, 28, and 52.
Statistical Analysis
Efficacy and safety analyses were assessed in the intent-to-treat population, stratified by sex and treatment arm. Analysis of the annual rate of on-treatment moderate/severe exacerbations was performed using a generalized linear model assuming a negative binomial distribution and covariates of treatment group, sex, exacerbation history (≤1, ≥2 moderate/severe), smoking status (screening), geographic region, postbronchodilator percentage predicted FEV1 (screening), and treatment group by sex interaction. Within the sex and treatment subpopulations, analysis of the annual rate of on-treatment moderate/severe exacerbations was performed, further stratified by age (<65 years, ≥65 years) and eosinophil count (baseline; <150 cells/µL, ≥150 cells/µL – a threshold supported in a previous systematic review and meta-analysis)25 using a generalized linear model assuming a negative binomial distribution and covariates of treatment group, exacerbation history (≤1, ≥2 moderate/severe), smoking status (screening), geographic region, postbronchodilator percentage predicted FEV1 (screening), and exacerbation history (<2 moderate and 0 severe in past year, ≥2 moderate or ≥1 severe in past year). Analyses of time to first on-treatment moderate/severe exacerbations were performed using a Cox proportional hazards model with covariates of treatment group, sex, exacerbation history (≤1, ≥2 moderate/severe), smoking status (screening), geographic region, postbronchodilator percentage predicted FEV1 (screening), and treatment group by sex interaction. Trough FEV1 and SGRQ data were analyzed using a repeated measures model (Weeks 4, 28, and 52), with covariates of treatment group, smoking status (screening), geographic region, visit, baseline, baseline by visit, and treatment group by visit interactions for each subgroup separately. All programming for statistical analyses was performed using SAS® Version 9.4 (SAS Institute Inc.; Cary, North Carolina).
Results
Patient Population
In total, 10,355 patients were included, comprising 6870 (66.3%) males and 3485 (33.7%) females. Overall, baseline characteristics were generally consistent between males and females (Table 1), with the exception that females had numerically higher baseline CAT and SGRQ scores (mean [standard deviation (SD)], CAT: 19.1 [7.13]; SGRQ: 52.4 [15.97]) compared with males (CAT: 17.8 [6.84]; SGRQ: 49.8 [17.24]). In addition, a greater proportion of females reported prior exacerbations at screening, including ≥2 moderate (n=1787 [51%]), ≥2 moderate/severe (n=2025 [58%]), and exacerbations treated with systemic/oral corticosteroids (n=1371 [39%]), compared with males (n=3090 [45%]; n=3630 [53%]; n=2415 [35%]; respectively). A similar proportion of males and females reported ≥1 severe exacerbation in the past year (n=1619 [23.6%] versus n=681 [19.5%]). The mean (SD) smoking pack-year history was higher for males (49.0 [28.04]), compared with females (41.9 [22.92]).
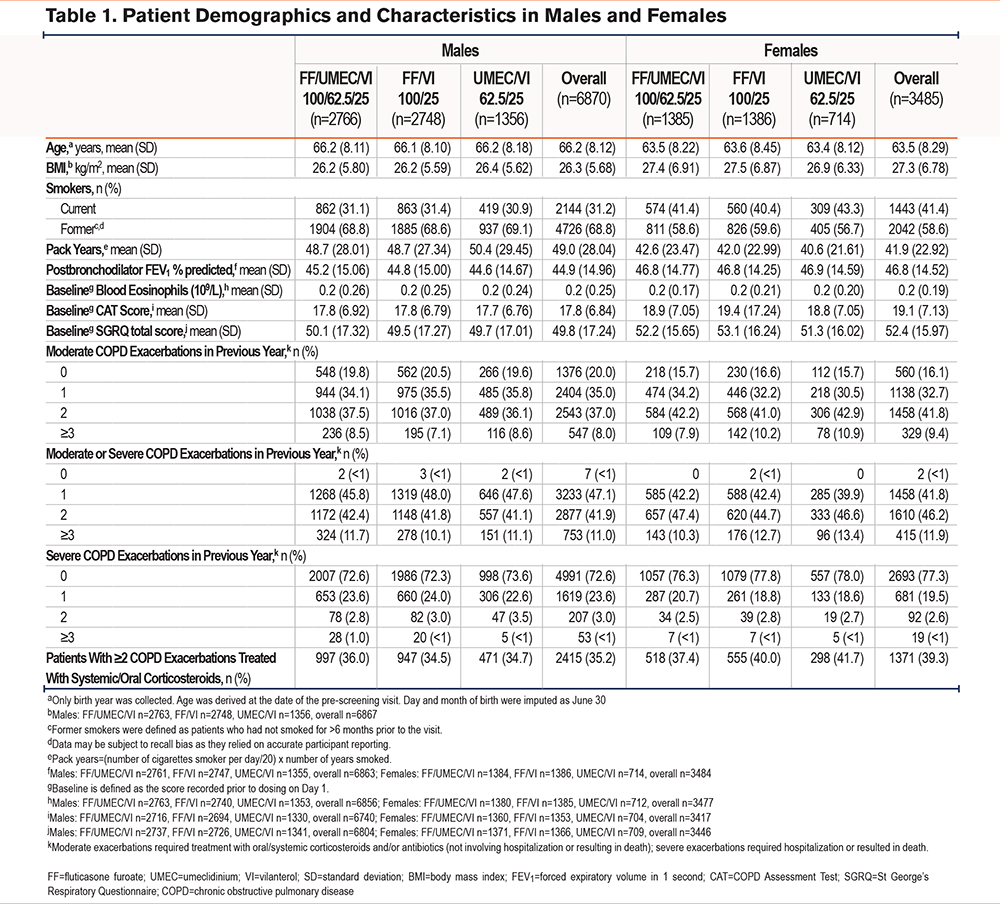
Efficacy Endpoints
COPD Exacerbations
The annual rate (95% confidence interval [CI]) of on-treatment moderate/severe COPD exacerbation rates for females remained higher across all treatment arms (FF/UMEC/VI: 0.99 [0.92, 1.07]; FF/VI: 1.19 [1.11, 1.29]; UMEC/VI: 1.35 [1.22, 1.49]), compared with males (FF/UMEC/VI: 0.87 [0.82, 0.91]; FF/VI: 1.01 [0.96, 1.07]; UMEC/VI: 1.14 [1.06, 1.23]), though overall exacerbation rates were significantly reduced for both sexes with FF/UMEC/VI compared with both dual therapies. In males, the reduction in the annual exacerbation rate was 14% (95% CI: 8%, 21%) for FF/UMEC/VI versus FF/VI and 24% (17%, 31%) for FF/UMEC/VI versus UMEC/VI; corresponding values for females were 17% (7%, 25%) and 26% (16%, 35%) (Figure 1).
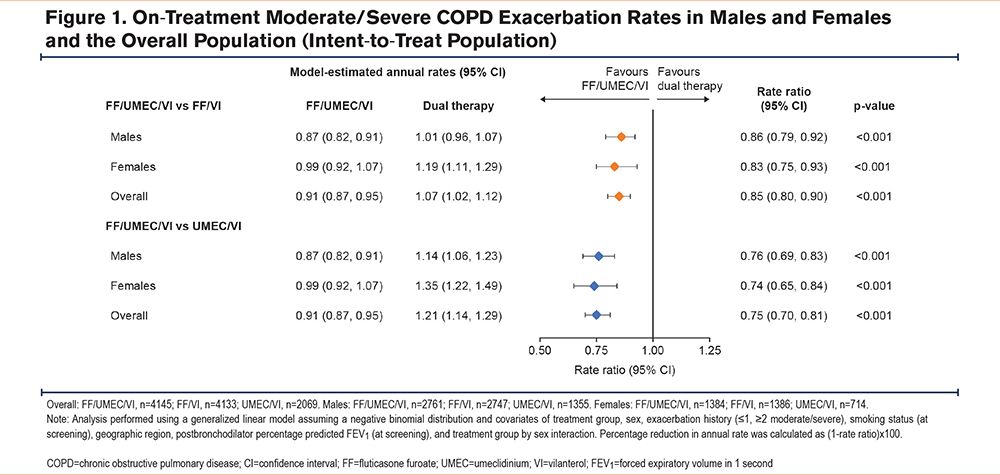
The proportion of patients with on-treatment moderate/severe COPD exacerbations remained higher for females across all treatments (FF/UMEC/VI: 51%; FF/VI: 52%; UMEC/VI: 56%), compared with males (FF/UMEC/VI: 45%; FF/VI: 48%; UMEC/VI: 47%); however, the reduction in risk (time-to-first) of a moderate/severe COPD exacerbation was significantly lower in both males and females with FF/UMEC/VI versus dual therapy; in males, the risk reduction was 16% (95% CI: 9%, 22%, p<0.001) for FF/UMEC/VI versus FF/VI and 13% (95% CI: 5%, 21%, p=0.003) for FF/UMEC/VI versus UMEC/VI and corresponding values for females were 13% (4%, 22%, p=0.006) and 20% (10%, 29%, p<0.001) (Figure 2).
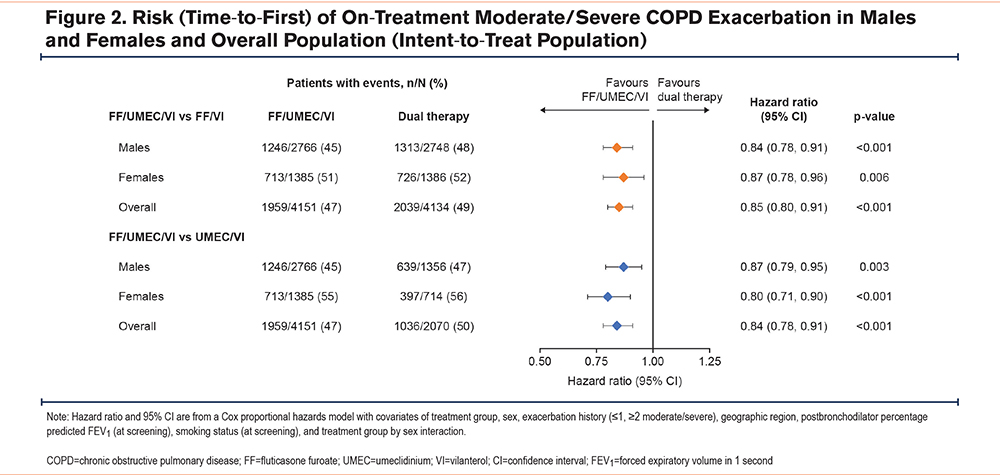
Overall, FF/UMEC/VI reduced the annual rate of on-treatment moderate/severe COPD exacerbations versus dual therapy when age, eosinophil count, and exacerbation history were considered, with greater responses seen in patients older than age 65 years across both sexes (Figure 3A). However, in patients with blood eosinophil counts <150 cells/µL, the annual exacerbation rate (95% CI) was significantly lower in females treated with FF/UMEC/VI (n=645) versus UMEC/VI (n=357) (0.81, [0.68, 0.97], p=0.024), but not in males (FF/UMEC/VI: n=1196, UMEC/VI: n=512; 0.92, [0.79, 1.08]) (Figure 3B). In patients with <2 moderate exacerbations and no severe exacerbations in the past year, the exacerbation rate was also significantly lower in females treated with FF/UMEC/VI (n=398) versus UMEC/VI (n=191) (0.73, [0.57, 0.94], p=0.013) but not in males (FF/UMEC/VI: n=799, UMEC/VI: n=425; 0.86, [0.72, 1.03]) (Figure 3C).
Lung Function
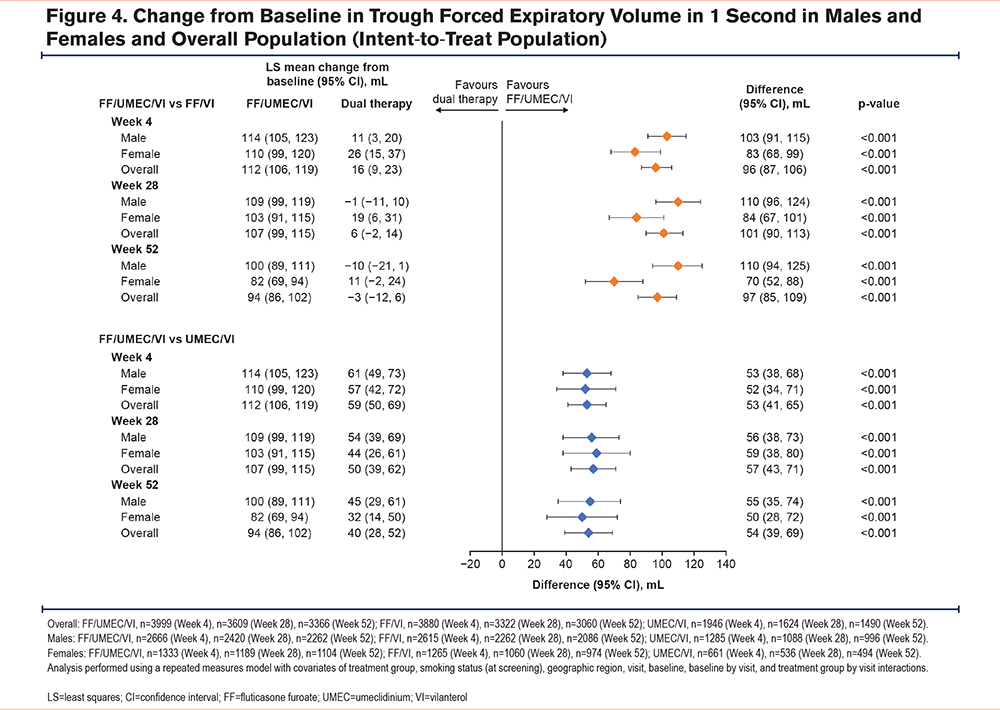
For both males and females, the mean improvement from baseline in trough FEV1 was significantly greater with FF/UMEC/VI versus FF/VI and UMEC/VI at all time points (Figure 4). However, in males, the difference in improvement across time points with FF/UMEC/VI versus FF/VI was 103–110 mL, whereas in females it was 70–84 mL (Figure 4).
Health Status
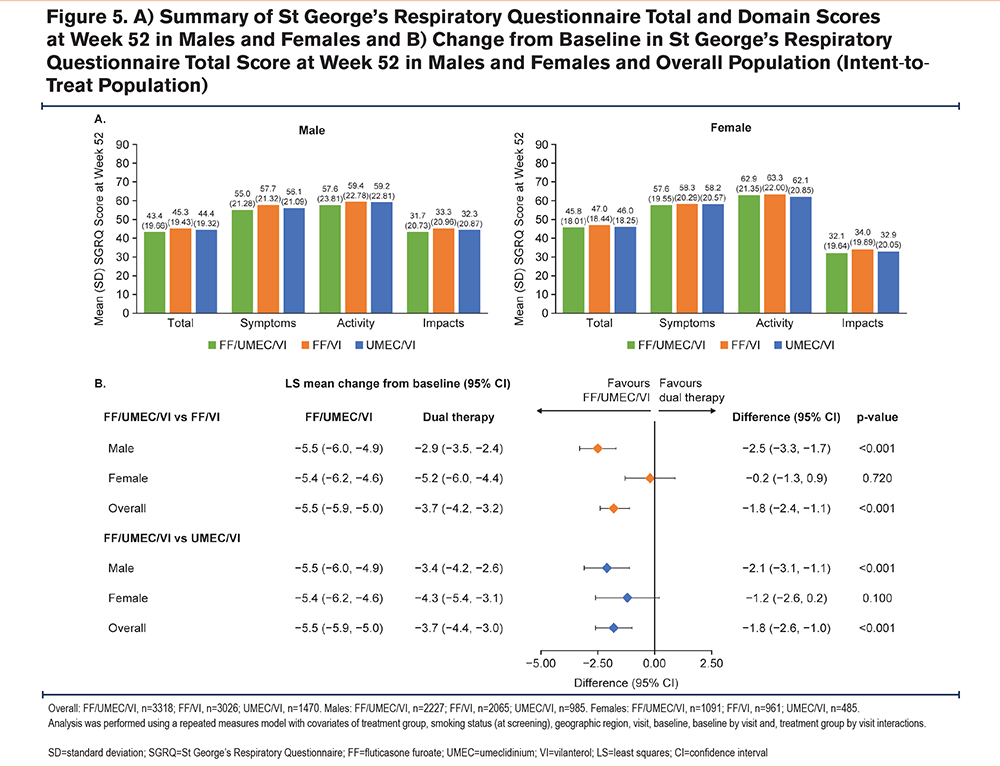
Mean SGRQ total and domain scores at Week 52 were similar in both males and females across treatments (Figure 5A). In males, improvements in SGRQ total score from baseline at Week 52 were significantly greater with FF/UMEC/VI compared with dual therapy (FF/UMEC/VI versus FF/VI: −2.5 [−3.3, −1.7], p<0.001; FF/UMEC/VI versus UMEC/VI: −2.1, [−3.1, −1.1], p<0.001) (Figure 5B). At Week 52, mean (SD) change from baseline in SGRQ domain scores was similar between males and females for FF/UMEC/VI (Total: −5.7 [14.55] versus −5.6 [13.73]; Symptoms: −9.7 [18.48] versus −9.1 [17.22]; Activity: −5.4 [17.58] versus −5.4 [16.33]; Impacts: −4.7 [16.83] versus −4.7 [16.22]).
Safety Endpoints
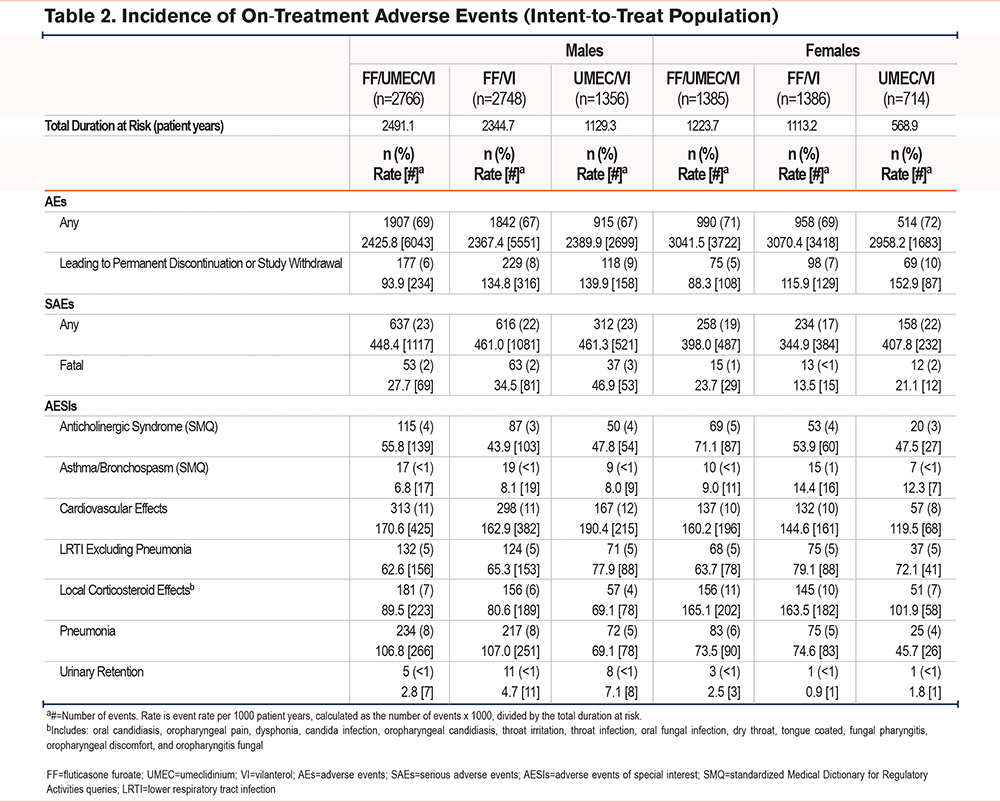
Males had a higher proportion of pneumonia events and higher exposure-adjusted rates of pneumonia per 1000 patient years compared with females across all treatment arms (FF/UMEC/VI: 8.5%, 106.8 per 1000 patient years versus 6.0%, 73.5 per 1000 patient years; FF/VI: 8.0%, 107.0 per 1000 patient years versus 5.4%, 74.6 per 1000 patient years; UMEC/VI: 5.3%, 69.1 per 1000 patient years versus 3.5%, 45.7 per 1000 patient years, respectively) (Table 2). Differences in the occurrence of local corticosteroid effects were also observed between males (FF/UMEC/VI: 7%, FF/VI: 6%, UMEC/VI: 4%) and females (FF/UMEC/VI: 11%, FF/VI: 10%, UMEC/VI: 7%). Females also had higher exposure-adjusted rates of local corticosteroid effects compared with males across all treatment arms (Table 2). The most common on-treatment AESIs were cardiovascular effects, occurring with a similar incidence and rate across all treatment groups among both males and females (8%–12% and 119.5–190.4 per 1000 patient years) (Table 2).
Discussion
This post hoc analysis of the IMPACT study demonstrated that FF/UMEC/VI treatment was effective at reducing exacerbations while improving lung function and health status in both male and female patients with COPD; however, some notable differences in clinical outcomes were observed between the sexes.
Female participants had a greater disease burden at baseline compared with males. Females reported a greater exacerbation history at screening compared with males, which is consistent with previous studies.9,11-14 In females, COPD exacerbations may be more commonly misinterpreted as symptoms of anxiety,26 and female patients with COPD are more likely to present with comorbid anxiety,4,15 which is associated with poorer health outcomes and increased exacerbation risk.26,27 There also appears to be a cyclical nature in the relationship between COPD and anxiety, where COPD increases the likelihood of anxiety, which in turn worsens COPD outcomes.26 Future studies should investigate the impact of sex-based differences in anxiety on treatment outcomes and its implications for clinical practice. The annual on-treatment rate and risk of exacerbations remained higher in females compared with males across treatment arms; however, reductions with FF/UMEC/VI versus dual therapy were observed among both male and female patients. In fact, the greatest exacerbation reduction was observed among females by the addition of FF to UMEC/VI. These data suggest that at least some of the increased exacerbation rates observed in females have a biological basis that is responsive to inhaled corticosteroid (ICS) therapy, as opposed to alternative explanations. There is a paucity of data regarding potential biological mechanisms regarding this, however, greater response to ICSs in COPD treatment in female over male patients has been reported previously in lung function improvements and reduced mortality.28 Though not directly linked to ICS response, hormonal differences, particularly regarding estrogen, have been proposed as a physiological mechanism indirectly affecting treatment outcomes,29 however, further research in this area is needed.
Despite a higher exacerbation rate compared with males, COPD is underdiagnosed in females,20 with a study reporting that a diagnosis of COPD was significantly more likely for the hypothetical male patient compared with the hypothetical female patient.6 Additionally, spirometry testing and pulmonologist referrals are less common for females.20 Subsequently, female participation in studies of COPD is typically lower than males. The greater disease burden identified in this study, as well as the underdiagnosis of COPD in females,3-5 highlights the need for efforts to increase female participation in future clinical trials of COPD.
Compared with males, we observed improved exacerbation rates with FF/UMEC/VI versus UMEC/VI in females with blood eosinophil counts <150 µL and those with <2 moderate and no severe exacerbations in the preceding year—patient groups traditionally thought to be at lower risk of exacerbation. While similar, nonstatistically significant trends were noted in males, the degree of exacerbation reduction and statistical significance was more pronounced in females when examining these subgroups. While we cannot know with certainty why this difference was observed, the data suggest that, generally speaking, females may be particularly sensitive to the benefits of ICSs, even beyond the subgroups traditionally identified as benefiting from ICS. Previous research has identified differences in blood eosinophil levels between males and females, with males typically having higher eosinophil counts compared with females, both in COPD and non-COPD populations.30-32 Thresholds for ‘high’ and ‘low’ eosinophil levels may, therefore, differ between the sexes when assessing treatment effect, and further research is needed to validate thresholds for male and female patients.
Treatment with FF/UMEC/VI improved lung function in both males and females compared with dual therapy at all time points. These results are consistent with the primary results of the IMPACT trial, which showed that the addition of the bronchodilator UMEC significantly improved lung function.22 The greater benefit observed in male patients in this analysis, as well as previously reported clinical trials,33,34 may indicate that clinicians should consider targeting treatment for shortness of breath in males because this may be due to differences in lung physiology between males and females.8 However, as the IMPACT trial did not collect data on the relative physiological differences between male and female patients to account for this observation, a causal mechanism could not be established. Further investigation of relative improvements in lung function between males and females with the addition of a long-acting muscarinic antagonist (LAMA) is warranted to expand understanding of sex-specific mechanisms of dyspnea. The addition of FF to UMEC/VI also improved lung function in patients, though the level of improvement did not differ between the sexes.
Health status scores were higher in females at baseline compared with males, indicating worse quality of life. Previous research has shown that female patients with COPD often have a worse health status compared with males,35 possibly due to the greater disease burden identified in females and misdiagnosis of anxiety.26 In males, FF/UMEC/VI demonstrated greater effects versus dual therapy for health status (as measured by SGRQ).
Overall, the incidence of AESIs was similar between males and females; however, males experienced numerically more pneumonia events whereas females experienced more local corticosteroid-related effects. Pneumonia incidence was higher in males compared with females, particularly in the ICS treatment arms (8.5% and 7.9% among males versus 6.0% and 5.4% among females in the FF/UMEC/VI and FF/VI treatment arms). A higher incidence of pneumonia is a class effect of ICS-containing therapies36; however, the benefit–risk of treatment with FF/UMEC/VI versus FF/VI or UMEC/VI has been demonstrated as favorable in a prespecified analysis of the IMPACT trial, with regards to the risk of combined pneumonia or moderate/severe exacerbations.37 Local corticosteroid effects were higher in females; however, discontinuation of an ICS has been associated with an increased probability of an adverse respiratory outcome in patients with COPD, with a higher risk among females.38
Limitations of this study include that all analyses were performed post hoc, and therefore, the study was not powered to assess sex differences. Formal statistical comparisons between males and females were not conducted because these differences may confound the results of any formal comparisons between the groups. Strengths of the study include a strong study design in the IMPACT study, which was a large prospective COPD clinical trial that was designed to be generalizable to clinical practice and contained a well-characterized patient population.
Conclusion
FF/UMEC/VI improved the exacerbation rate, lung function, and health status compared with FF/VI and UMEC/VI in patients with symptomatic COPD and a history of exacerbations, regardless of patient sex. However, sex differences were identified, including greater disease burden among females, indicated by greater exacerbation history and worse health status at baseline compared with males. The on-treatment rate and risk of moderate/severe COPD exacerbations were also higher among females compared with males. Males experienced greater improvements in lung function with FF/UMEC/VI versus FF/VI compared with females, suggesting a potential greater benefit of LAMA addition in males. However, female patients with characteristics thought to be at lower risk of exacerbations, with eosinophil counts <150 cells/µL and <2 moderate and no severe exacerbations in the preceding year, experienced exacerbation reduction with FF/UMEC/VI treatment compared with UMEC/VI that was not seen in males. Because baseline exacerbation rates were higher in female patients, this suggests a high steroid response in females across a range of patient characteristics.
This post hoc analysis highlights differences in the presentation of COPD between males and females. While FF/UMEC/VI is beneficial in the treatment of COPD regardless of patient sex, these data do emphasize the importance of individualized patient discussions. Furthermore, these data underscore the importance of personalized treatment approaches for both males and females with regard to safety as well as efficacy.
Acknowledgements
Author contributions: AHA, NBN, ABM, and DAL contributed to study concept/design and data interpretation. LT contributed to data analysis and data interpretation. MKH contributed to data interpretation. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Data sharing statement: Please refer to the GSK weblink to access GSK’s data sharing policies and as applicable seek anonymized participant level data via the link https://www.gsk-studyregister.com/en/.
Editorial support in the form of preparation of the first draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, was provided by Alexandra Berry, PhD, and Catherine Widnall, PhD, of Fishawack Indicia Ltd, United Kingdom, part of Avalere Health, and was funded by GSK (CTT116855/NCT02164513). ELLIPTA is owned by or licensed to the GSK Group of Companies.
Declaration of Interest
AHA, NBN, ABM, and DAL are employed by and hold financial equities in GSK. LT is a contingent worker on assignment at GSK. MKH has received either in-kind research support or funds paid to the institution from the National Institutes of Health, Sanofi, Novartis, Nuvaira, Sunovion, Gala Therapeutics, the COPD Foundation, AstraZeneca, the American Lung Association (ALA), Boehringer Ingelheim, and Biodesix; consulting fees from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Altesa BioSciences, Amgen, Roche, United Therapeutics, RS Biotherapeutics, and Apreo Health. She has received royalties from UpToDate, Norton Publishing, and Penguin Random House. She has received payment or honoraria for consultancy from Cipla, Chiesi, AstraZeneca, Boehringer Ingelheim, GSK, Medscape, Integrity, NACE, and Medwiz. She has served roles on boards or scientific committees for the COPD Foundation Board, the COPD Foundation Medical and Scientific Advisory Committee, the ALA advisory committee, and has been an American Thoracic Society journal editor, an ALA volunteer spokesperson, and on the Global Initiative for Chronic Obstructive Lung Disease Scientific Committee and the Emerson School Board, Ann Arbor, Michigan. She has participated in data safety monitoring boards for Novartis and Medtronic, with funds paid to the institution. She holds stock options from Meissa Vaccines and Altesa BioSciences.