Running Head: Pharmacist Inhaler Prescribing for COPD
Funding Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Date of Acceptance: November 13, 2024 | Publication Online Date: November 26, 2024
Abbreviations: AECOPD=acute exacerbation of chronic obstructive pulmonary disease; ALF=assisted living facility; BMI=body mass index; CMS=Centers for Medicare and Medicaid Services; COPD=chronic obstructive pulmonary disease; EMR=electronic medical record; GDMT=guideline-directed medical therapy; GOLD=Global initiative for chronic Obstructive Lung Disease; HHC=home health care; HRRP=Hospital Readmissions Reduction Program; ICS=inhaled corticosteroid; IRF=inpatient rehabilitation facility; LABA=long-acting beta2- agonist; LAMA=long-acting muscarinic antagonist; RN=registered nurse; RPh=registered pharmacist; SD=standard deviation; SNF=skilled nursing facility; TOC=transitions of care
Citation: Diaz AM, Smith LM, Peterson AN, Kent ML, Vellian NJ. Impact of pharmacist inhaler prescribing at discharge for chronic obstructive pulmonary disease on readmission rates. Chronic Obstr Pulm Dis. 2025; 12(1): 43-51. doi: http://doi.org/10.15326/jcopdf.2024.0553
Introduction
The Global initiative for chronic Obstructive Lung Disease (GOLD) 2024 guideline reports chronic obstructive pulmonary disease (COPD) as one of the top 3 causes of death worldwide, affecting one in 10 adults globally.1 In the United States alone, COPD accounts for nearly 700,000 hospitalizations each year and an estimated annual health care cost of $50 billion, with the greatest proportion of this expense attributed to the treatment of acute exacerbation of COPD (AECOPD).2
AECOPD is the third leading cause of readmissions in the United States, with a national average of 20% of patients readmitted within 30 days of discharge,2 although many individual institutions have reported rates3 as high as 40%. Treatment for AECOPDs requiring hospitalization consists of supplemental oxygen, systemic corticosteroids, antibiotic therapy when indicated, short-acting bronchodilators, and triple therapy at discharge, which includes an inhaled corticosteroid, a long-acting muscarinic antagonist (LAMA), and a long-acting beta2-agonist (LABA).1 Triple therapy may be prescribed in the form of inhalers or nebulizer solutions, as trials have found that both are equally effective in patients with proper inhalation device technique.4
In October 2014, AECOPD was included in the Centers for Medicare and Medicaid Services (CMS) Hospital Readmissions Reduction Program (HRRP), alongside myocardial infarction, heart failure, pneumonia, coronary artery bypass graft, and elective total hip and knee arthroplasty.5 The HRRP assesses hospital readmission data for these 6 high-impact conditions and enforces up to a 3% payment penalty to institutions with 30-day readmission rates above a predetermined benchmark set by CMS, regardless of readmission reason.
Existing strategies to reduce AECOPD readmissions include medication reconciliation at discharge by a pharmacist, postdischarge phone calls, pulmonology appointments within 30 days of discharge, personalized COPD action plans, patient education, counseling, and the use of triple therapy inhalers.6 In a recent study, Lipson et al compared triple therapy inhalers versus dual therapy (LABA/LAMA) in AECOPD patients and found a 25% reduction (p<0.001) of moderate to severe exacerbations and a 34% decrease (p<0.001) in the rate of hospitalization with triple therapy.7 The GOLD guidelines endorse triple therapy as an effective strategy to reduce COPD readmissions; however, the utilization may be limited due to various medication barriers, such as the high cost of inhalers.1
Current literature has introduced the role of transitions of care (TOC) pharmacists in the reduction of readmission rates with varied results. Kim et al8 compared TOC pharmacy services to usual care in patients with COPD, demonstrating a lower 30-day readmission rate of 2% in the intervention group compared to 17.3% in usual care (p=0.009). In this study, the pharmacy service provided medication recommendations to physicians and followed up with patients via phone or clinic appointments.8 On the contrary, a 2024 retrospective evaluation of a TOC pharmacy service did not show a significant reduction in readmission rates.9 Services included patient counseling, medication reconciliation, medication access assistance, and therapy optimization with physician approval. The lack of improvement in the intervention group may be attributed to the poor intervention acceptance rate by providers (58%), thus reinforcing the notion that provider collaboration with pharmacists is imperative to the success of reducing readmissions and optimizing patient outcomes.9
In 2021, an internal review at this study site analyzed the 30-day readmission reason for patients who were initially admitted for AECOPD and found that nearly 72% of patients were readmitted with a subsequent AECOPD. This evaluation also revealed that only 30% of patients were discharged on guideline-directed inhalers during their index visit. In June 2022, with increased challenges leading to continued high readmission rates for COPD, the pulmonologists at the study site expressed interest in collaborating with the TOC pharmacists to improve overall prescribing of inhalers at discharge. To achieve this goal, a hospital-approved protocol was created to grant authority for the TOC pharmacists to prescribe triple therapy inhalers at discharge. This protocol ensures patients were discharged on guideline-directed medical therapy (GDMT) consisting of a rescue plus triple therapy inhalers.
Despite growing evidence for the integration of TOC pharmacy models into hospital practice, preceding studies have not yet identified a single pharmacy intervention that consistently demonstrates the reduction of readmissions. Currently, there is a lack of literature with respect to pharmacists prescribing inhalers upon hospital discharge under an approved protocol. The purpose of this study was to evaluate all-cause 30-day COPD readmission rates.
Study Design and Methods
This was an institutional review board-approved (Lakeland Regional Health Institutional Review Board-IRB00002126), single-center, retrospective cohort evaluation conducted between May 2021 and August 2023. This institution is an integrated health care system comprised of one medical center and 19 community-based clinics. The medical center is a 910-bed, not-for-profit, teaching hospital that serves as the sole hospital in a medically-underserved area providing care to a county of approximately 780,000 residents with a ratio of one primary care doctor for every 2170 patients. The pharmacists utilize a collaborative team approach with providers, particularly with the internal medicine service comprised of 132 hospitalists and a pulmonology service of 10 physicians and 2 nurse practitioners.
Historically, COPD readmission rates at this study site exceeded the predetermined HRRP benchmark of 19.6%, with an average of 23.7% for fiscal year 2022. The dedicated HRRP readmission team involved 2 inpatient TOC clinical pharmacists and 3 nurse care coordinators. The nurses completed postdischarge calls and educated the patient while the pharmacists focused on medication interventions. In attempts to mitigate readmission penalties, this institution created a hospital-approved standard operating procedure granting the TOC pharmacists prescribing authority to send a 30-day supply of a rescue and triple therapy inhaler(s) at discharge for AECOPD patients.
The TOC pharmacists provided additional recommendations for therapeutic interventions, although not all of these suggestions were consistently accepted by the provider(s). In addition to the protocol, the TOC pharmacists optimized overall treatment regimens during admission and at discharge. Interventions included but were not limited to: streamlining antibiotics, adding a steroid taper at discharge for patients on high-dose steroids during admission, renally dose adjusting medications, adjusting insulin doses, providing patient education, and reviewing medication reconciliations for discrepancies, therapeutic duplications, or contraindications.
Patient Selection
This study evaluated pre- and postimplementation of the TOC pharmacy protocol created in June of 2022. The pre-implementation group included patients discharged between May 1, 2021, and May 31, 2022, for their index admission and the postimplementation group were discharged between June 1, 2022, and August 31, 2023.
All patients who met criteria for the HRRP COPD model were included: aged 65 years and older, a principal diagnosis of AECOPD or acute respiratory failure with AECOPD as the secondary diagnosis, alive at discharge, enrolled in Medicare Fee-for-Service Parts A and B for at least 12 months prior to the date of admission, and for Veteran’s beneficiaries concurrently enrolled in Medicare Fee-for-Service Part A. Along with meeting the aforementioned criteria, the postimplementation group must also have been enrolled in the TOC pharmacy service and originally discharged by the provider without triple therapy inhalers. Patients were excluded if diagnosed with COVID-19, readmitted with COVID-19, left against medical advice, or were discharged to a long-term acute care hospital. An additional exclusion was applied to the pre-implementation group if enrolled in the TOC pharmacy service. Patients were included more than once if 30 or more days passed between admissions.
Description of Intervention
The TOC pharmacy service was a multifaceted intervention that aimed to improve clinical outcomes and readmission rates for Medicare patients (Figure 1). TOC pharmacists identified patients who met the HRRP COPD model criteria via a report from the electronic medical record (EMR), performed a thorough and accurate medication history, and ensured the admission medication reconciliation was completed within 24 hours of admission. Throughout the inpatient admission, patient charts were reviewed daily to determine if therapeutic interventions were warranted, regardless of whether they were within protocol or required provider approval. Inpatient interventions included clarifying duplicate therapy, antibiotic stewardship such as streamlining antibiotics and/or renal dose adjustment, tapering steroids and converting from intravenous to oral formulations, resuming home medications at the right dose and frequency, and adjusting insulin regimens. Once a discharge order was placed, the TOC pharmacist received an email and reviewed the discharge medication reconciliation to identify discrepancies. COPD treatment regimens were reviewed by the pharmacist who ensured GDMT was prescribed at discharge and confirmed all pulmonology recommendations were executed. If GDMT was not prescribed at discharge, the pharmacist prescribed the appropriate inhaler(s) and discontinued duplicate inhaler therapy per protocol. To increase patient adherence, a single combination triple therapy inhaler such as umeclidinium/vilanterol/fluticasone furoate or budesonide/formoterol/glycopyrrolate was most frequently prescribed. Combinations of single and dual inhalers or nebulized solutions such as arformoterol, budesonide, and ipratropium were prescribed for patients with formulary or cost restrictions. The pharmacist contacted the retail pharmacy to ensure affordability and applied free trial cards from the manufacturer or other available coupons as needed. Pharmacists regularly collaborated with the pulmonology service on complex patients to determine appropriate therapy optimization based on specific patient factors. For patients without Medicare Part D, the pharmacists prescribed nebulized solutions billed through Part B or contacted the consulting pulmonologist to provide inhaler samples until a long-term plan could be determined. The team also allied with case management and nursing to ensure patients received a nebulizer machine prior to discharge. For patients who discharged outside of coverage hours, medication regimens were reviewed and adjusted the following business day.
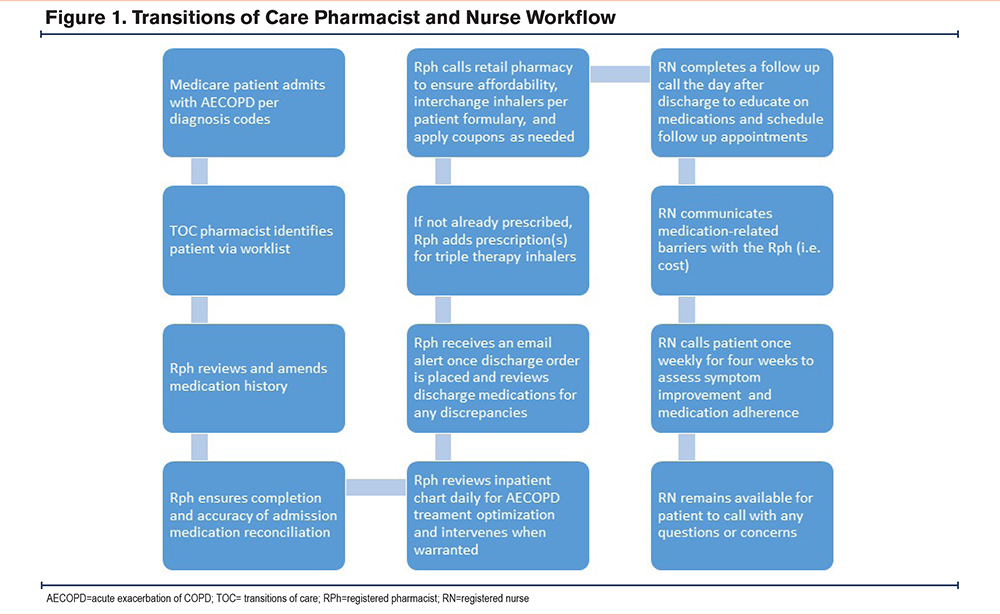
The postdischarge nurse coordinator service was available to call patients who were discharged home the following business day. This service provided extensive education of the disease state, medications, inhaler technique, and smoking cessation strategies. The nurse called the patient weekly for 4 weeks postdischarge, assisted in scheduling follow-up appointments with primary care and pulmonology, and assessed symptom improvement. The nurse team communicated closely with the pharmacist in the event patients needed to transfer prescriptions to a different pharmacy or identified challenges in obtaining medications following discharge.
Outcomes
The primary outcome of this study was all-cause 30-day COPD readmission rates. Hospital visits were evaluated by chart review, and readmissions were counted if the patient was admitted as inpatient status to this institution within 30 days of discharge. Secondary outcomes included readmission reason (COPD versus other) and the proportion of patients discharged on GDMT. If upon readmission the patient required any acute treatment for AECOPDs (i.e., steroids, bronchodilators, antibiotics), the readmission reason was reported as COPD.
Data Collection and Statistical Analysis
Baseline characteristics were analyzed using descriptive statistics, and outcomes were compared using Chi-square or Fisher's exact tests. A power calculation was performed after implementation of the TOC service. The estimated sample size needed was 240 admissions (power = 0.80 with a 2-sided alpha of 0.05) to detect a 50% reduction in the postimplementation group readmission rate, presuming the readmission rate was previously 30%. A p-value <0.05 was considered to indicate statistical significance.
Results
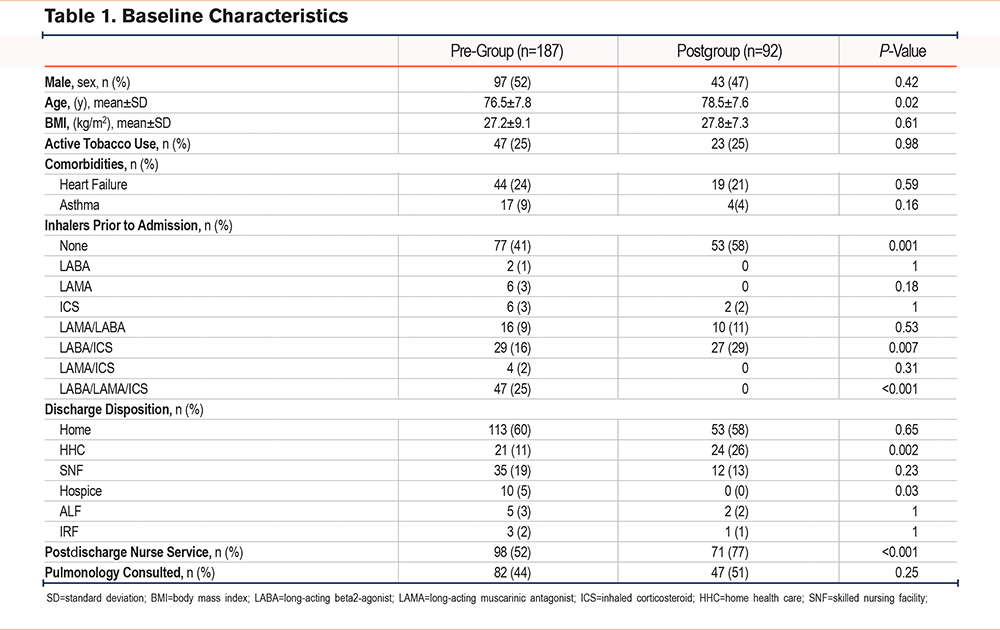
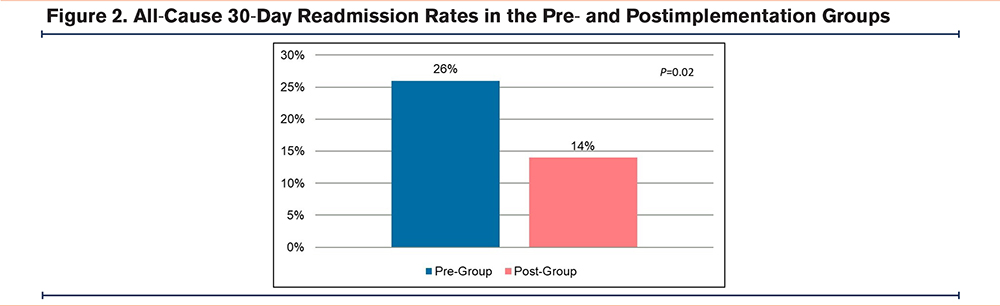
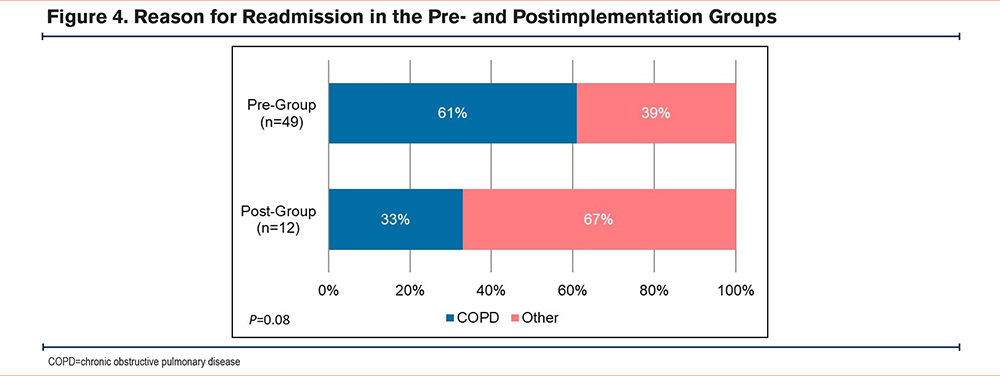
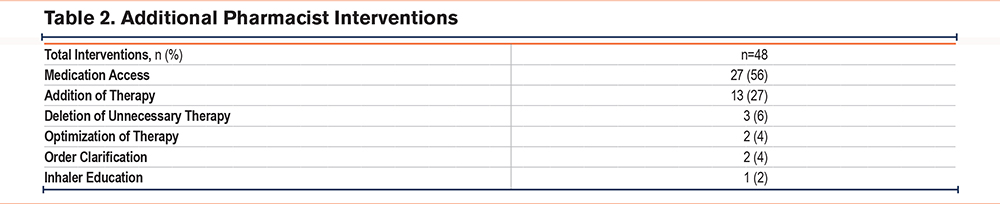
A total of 279 patients met inclusion criteria, with 187 patients in the pre-implementation group and 92 patients in the postimplementation group. There were a few significant differences in baseline characteristics between the 2 cohorts. A higher percentage of patients in the postimplementation group received the postdischarge nurse service, were on no maintenance inhalers prior to admission, and discharged home with home health care (Table 1). All-cause 30-day readmission rates in the pre- and postimplementation groups were 26% and 14%, respectively, resulting in an absolute risk reduction of 12% and a relative risk reduction of 46% (p=0.02) (Figure 2). The proportion of patients discharged on GDMT was 26% in the pre-implementation group and 100% in the postimplementation group (p<0.001). In the pre-implementation group, 17% of patients were discharged with only a rescue inhaler and no maintenance inhalers, while 9% were discharged with no inhalers at all (Figure 3). In regards to readmission reason, AECOPD accounted for 61% in the pre-group compared to 33% in the postgroup (p=0.08) (Figure 4). Reasons for readmissions in the postimplementation group included: gastrointestinal infections, falls with fractures, thrombocytopenia, skin infection, chronic pain, pulmonary fibrosis, and pleural effusion. Additional pharmacist interventions primarily focused on assisting patients with affordability of their medications (Table 2). There were 24 patients in the postimplementation group who could not afford their discharge inhalers. On average, the pharmacists saved each patient about $255 by utilizing free trial cards or switching to alternative inhalers.
Discussion
This TOC pharmacy initiative was, to our knowledge, the first study to report an approved protocol for pharmacists to prescribe inhalers at discharge. This strategy was associated with a statistically significant reduction in 30-day readmission rates after the implementation of a TOC pharmacy service, compared to readmission rates in the pre-implementation period. This multidisciplinary approach decreased the hospital’s all-cause 30-day readmission rate to well below the benchmark goal of 19.6%, mitigating readmission penalties. Patients were also less likely to be readmited with a recurring COPD exacerbation in the postimplementation group. Although this reduction was not statistically significant, it upholds the concept that prescribing GDMT at discharge reduces the risk of subsequent exacerbations.
Preceding literature substantiates the benefits of pharmacist-led interventions for COPD patients at discharge; however, the impact of therapeutic interventions in these studies was limited by poor acceptance rates by providers. A recent study10 evaluated the outcomes of a pharmacist-driven COPD TOC bundle where the pharmacists were required to obtain provider acceptance for interventions, of which 81% were accepted. Although this rate exhibited a promising future for provider collaboration compared to previous studies, intervention acceptance rates remain a limiting factor in pharmacists optimizing GDMT.10 Our study allowed pharmacists to work under an approved protocol, therefore bypassing the need for physician approval and creating a more streamlined process for the pharmacist to select patient-specific inhalers. As a result, 100% of patients in the postimplementation group were discharged on GDMT.
Access to medications is another major barrier for patients with COPD that could lead to readmission. Medication adherence is critical to manage symptoms and prevent future exacerbation risk. In a national survey of adults with COPD from 2013–2020, one in 6 adults reported experiencing cost-related medication nonadherence, with 12.5% missing doses, 13.3% taking lower than prescribed doses, and 15.74% delaying filling prescriptions to save cost.11 The TOC pharmacists were meticulous in selecting and interchanging inhalers until a regimen was affordable and convenient for the patient. In addition to confirming prices, the pharmacists would ensure the selected inhalers were in stock at the pharmacy, especially for discharges on Fridays, to avoid missed doses. These extra measures to ensure medication adherence and patient satisfaction would often take the pharmacists several hours and multiple phone calls to accomplish. Providers may not have the time or capacity to repeatedly call pharmacies, further illustrating the advantages of pharmacy involvement in inhaler selection.
Although this study did not evaluate financial implications through a cost analysis, the results demonstrate a very promising opportunity for cost reductions in hospitals with high readmission rates that would profoundly offset any expenses of the TOC pharmacy service. This study strongly suggests that mimicking this model at other institutions could potentially avoid monetary penalties from Medicare; however, fiscal exploration may help support and refine these findings.
Limitations and Challenges
There were notable limitations to this study, including its retrospective cohort design, use of the EMR, which does not capture readmissions to all other health care facilities, and limited data collection points due to inconsistent documentation. Although the EMR did not capture visits to other hospitals, the postdischarge nurse service was largely made aware of readmissions to outside institutions when carrying out follow-up calls. Due to the isolated location of our study site, we generally find patients readmited back to our institution; however, there may have been outliers. COPD severity and history of exacerbations were not routinely documented for all patients and, therefore, were not included as baseline characteristics. This could lead to bias, as we were unable to determine if one group had more severe COPD than the other. Collection of the prior to admission inhalers was dependent on documentation of the home medications. Medication histories were primarily completed by nursing until late 2021, when a pharmacy medication history technician program was implemented. As a result, the accuracy of prior to admission inhaler documentation in the pre-group was potentially lower than the postgroup. Patients in the pre-group may have seemingly discharged on maintenance inhalers, although they may not have had access to these medications due to cost or lack of refills. While this may be identified as a potential confounder, only 26% of patients in the pre-group discharged on GDMT, suggesting that regardless of the medication history accuracy, inhaler regimens were not consistently adjusted or escalated by providers. It should be noted that although the medication histories were potentially more accurate in the postgroup, providers did not correctly adjust inhaler regimens for any of these patients leading the pharmacists to utilize their protocol to prescribe GDMT.
The TOC pharmacists utilized a feature within the EMR to produce a list of patients with traditional Medicare insurance and AECOPD in their problems and diagnoses list. Challenges arose when the diagnosis codes or insurance were documented inaccurately. In order for patients to count for the HRRP readmission program, they must have a final coding summary of AECOPD as their principal discharge diagnosis or acute respiratory failure with AECOPD as the secondary diagnosis. Several patients who were included in the service ended up not counting for the HRRP program as the final coding was billed with a principal discharge diagnosis other than AECOPD or acute respiratory failure with a secondary of AECOPD. On the other hand, some patients who were originally excluded from the service due to having nontraditional Medicare in the chart turned out to actually have traditional Medicare and were missed from the service. Because coding is not finalized until after discharge, the TOC pharmacists needed to follow all patients who may count, which exerted additional resources and time.
The postdischarge nurse service 30-day enrollment was limited to following patients discharged to home who agreed to the service. As a result, we were unable to assess adherence to medications for patients discharged to skilled nursing facilities, inpatient rehabilitation, or who denied the nurse service. We also found challenges in patient engagement in discharge calls, as some may have enrolled in the service during the first follow-up call but later declined services or did not answer calls. Patients who were not enrolled in the postdischarge nurse service missed opportunities for assistance with scheduling follow-up pulmonology appointments as well as extensive medication education and counseling.
Future Directions
Since completion of this study, the protocol has been expanded to include additional prescribing opportunities for pharmacists to optimize therapy for AECOPD at discharge. TOC pharmacists can now prescribe a steroid taper for patients on high-dose steroids, defined as a total daily dose of prednisone 80mg or equivalent, as well as albuterol and ipratropium nebulized solutions as needed for shortness of breath. Since the initiation of the protocol in 2022, the TOC pharmacists have prescribed inhalers for more than 200 patients.
Further research is warranted to explore the role of TOC pharmacists in managing COPD among younger individuals and those not insured by traditional Medicare. Including additional patient populations in the analysis will provide a more comprehensive understanding of how pharmacists can effectively enhance COPD outcomes across diverse demographics. In the coming years, the TOC pharmacy service at this institution anticipates broadening services to non-HRRP patients.
Conclusion
Utilizing a multidisciplinary team of TOC pharmacists with an inhaler prescribing protocol and postdischarge nurse follow-up may be associated with a reduction in all-cause 30-day readmission rates for patients with COPD in a Medicare population. The findings of this study further validate the importance of prescribing GDMT upon discharge while also displaying the significance of the role of TOC pharmacists in reducing COPD readmissions.
Acknowledgements
Author contributions: AD is the guarantor of the content of the manuscript, including the data and analysis and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. AD, LS, and AP were responsible for the design. LS and AP were responsible for the conceptualization. AD, LS, and AP were in charge of the implementation. AD was responsible for the institutional review board approval and data collection. AD, LS, and MK were in charge of the data analysis. AD, LS, MK, NV, and AP contributed to the manuscript development and edits. AD was responsible for the manuscript submission. All authors read and approved the final version of the manuscript submitted for publication.
Declaration of Interests
The authors have no conflicts of interest or financial relationships to disclose.