Running Head: Statin and Aspirin Effects on Muscle in Smokers
Funding Support: The COPDGene study (NCT00608764) is supported by National Heart, Lung, and Blood Institute U01 HL089897 and U01 HL089856, as well as by the COPD Foundation through contributions made to an Industry Advisory Board comprised of AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Date of Acceptance: November 25, 2024 | Publication Online Date: December 4, 2024
Abbreviations: 6MWD=6-minute walk distance; ACEI=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; BMI=body mass index; CT=computed tomography; COPD=chronic obstructive pulmonary disease; COX=cyclooxygenase; DBP=diastolic blood pressure; FEV1=forced expiratory volume in 1 second; FEV1%pred=forced expiratory volume in 1 second percentage predicted; GERD=gastroesophageal reflux disease; GOLD=Global initiative for chronic Obstructive Lung Disease; HU=Hounsfield units; IQR=interquartile range; PGE2=prostaglandin E2; PMA=pectoralis muscle area; PMD=pectoralis muscle density; PSM=propensity score matching; SAM=statin-associated myopathy; SBP=systolic blood pressure; SE=standard error
Citation: Shirahata T, Enzer NA, Castro V, et al. Effect of common medications on longitudinal pectoralis muscle area in smokers. Chronic Obstr Pulm Dis. 2025; 12(1): 23-32. doi: http://doi.org/10.15326/jcopdf.2024.0557
Online Supplemental Material: Read Online Supplemental Material (164KB)
Introduction
Cigarette smoking is arguably one of the most significant health risk behaviors in our society. Although the prevalence of smoking among adults aged over 18 years in the United States has declined from 20.9% in 2005 to 12.5% in 2020, estimates suggest that 30.8 million adults are still current smokers and more than 16 million Americans live with a smoking-related disease.1,2 Importantly, cigarette smoke contributes to the development of many chronic diseases, including skeletal muscle wasting and dysfunction.3-5
There are a variety of approaches to measuring muscle loss, such as dual-energy X-ray absorptiometry, bioelectrical impedance analysis, ultrasound, and magnetic resonance imaging.6-8 Body composition is generally measured by these methods but our research and that of others has shown chest computed tomography (CT) scans can be used to obtain a good approximation of fat-free mass index.9,10 In previous studies, the CT cross-sectional area of the pectoralis muscles has been reported to be independently associated with energy expenditure, disease severity, and prognosis in smokers both with and without chronic obstructive pulmonary disease (COPD).10-14 Given the recommendations for CT lung cancer screening in current and former smokers with a significant smoking history, one area of interest has been the secondary use of these images to identify extrapulmonary diseases such as muscle loss.15
Although prior research has consistently shown an association between skeletal muscle loss and numerous adverse outcomes in smokers, especially those with COPD, limited interventions exist to directly target the loss of muscle mass.16,17 For example, the American Thoracic Society and European Respiratory Society recommend pulmonary rehabilitation and nutritional supplement for patients with chronic lung disease to improve exercise capacity, reduce dyspnea, increase muscle volume, and enhance health-related quality of life.18 While the identification of novel agents to treat muscle loss may be of longer-term interest, it is possible that medications commonly used for other indications in this at-risk population may prevent or attenuate the loss of skeletal muscle. In this study, we aimed to identify specific medications affecting skeletal muscle change as assessed by CT imaging using data from the COPD Genetic Epidemiology (COPDGene®) study.19 We hypothesized that medications used for the treatment of conditions common in smokers would be associated with longitudinal skeletal muscle loss. We further hypothesized that the medication effect would be dependent on persistent long-term use of the medication.
Material and Methods
Study Population and Design
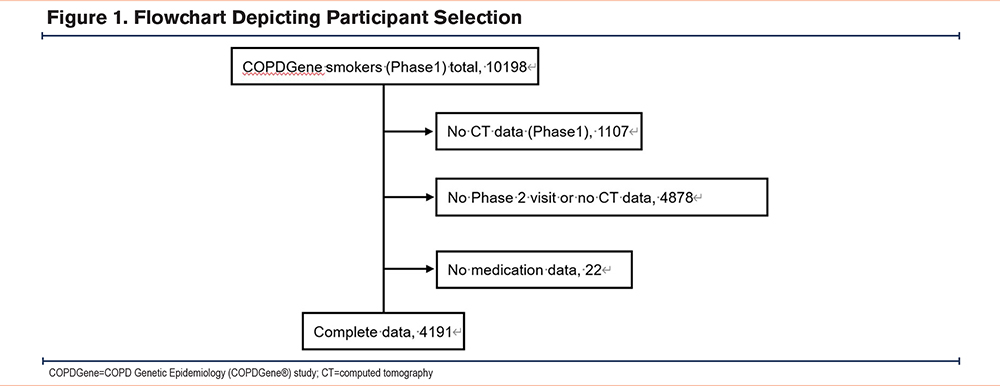
The COPDGene study have been previously described in detail.19 In brief, the COPDGene study (Clinicaltrials.gov identifier NCT00608764), is ongoing and enrolled a total of 10,198 non-Hispanic White and African American ever smokers from 21 centers in the United States, including those with and without COPD. The participants were aged 45–80 at enrollment and had a history of at least 10 pack years of smoking. The institutional review board of each center approved the study and written informed consent was obtained from all participants. Each participant’s assessments were conducted approximately every 5 years. All participants underwent anthropometric measurements, lung function tests, a 6-minute walk distance (6MWD) test, and a chest CT at each visit. In addition, the incidence of exacerbations and prescription data at the baseline and follow-up visits were recorded. Spirometry was performed before and after the administration of a bronchodilator. Diagnosis and severity of COPD were defined according to the Global initiative for chronic Obstructive Lung Disease (GOLD)20 guidelines with a postbronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio less than 0.7. In this study, we utilized COPDGene data from their first (phase 1) and second (phase 2) visits, which were an average of 5 years apart. The dataset included 4191 participants who had reported medication and chest CT scan data available from both visits. The details of the assembly of the cohort are presented in the diagram detailed in Figure 1.
Imaging Analysis
We used an established protocol to estimate CT-derived muscle measurements which has been described in detail in previous reports.12,13 In brief, we measured the cross-sectional pectoralis muscle area (PMA) from a single axial slice of chest CT at the top of the aortic arch using a deep learning approach to select the CT slice21 and a uNet-based network to segment the pectoral muscles22,23 and implemented in the Chest Imaging Platform.24 In addition, we measured pectoralis muscle density (PMD) in the areas where PMA was measured, as PMD is considered to reflect muscle quality and physical function.25 The PMA and PMD were defined as the sum and mean attenuation of the right and left pectoralis major and minor muscles, expressed in centimeters squared (cm2) and Hounsfield units (HU), respectively.
Statistical Analysis
All statistical analyses were performed using JMP pro version 16 software (SAS Institute Inc.; Cary, North Carolina). Univariate linear regression analysis was performed to examine the relationship between the change in PMA and prescribed medications. Multivariable regression analysis was performed to identify independent associations between delta-PMA and medications using the medications with p-value <0.2 from the univariate regression analyses.26 The change in PMA/PMD (delta-PMA/PMD) is defined as PMA/PMD at phase 2 – PMA/PMD at phase 1. Prescribed medications that affect body composition were tested in the models if they were reported or reportedly prescribed for over at least 5% of the study population. Data observed were not normally distributed and nonparametric statistical methods were used for statistical analysis. Data pertaining to the continuous variables were described in the median and interquartile range (IQR). The Wilcoxon test was used for group comparison, and multiple comparison was performed with the Kruskal-Wallis test followed by the Steel test. Proportional data were analyzed using the Chi-squared test.
We carried out a propensity score matching (PSM) analysis using a logistic regression procedure to determine the effects of medications after matching age, gender, race, body mass index (BMI), FEV1 percentage predicted (FEV1%pred), smoking status, pack years, comorbidities, and the use of independently associated medication except for the index medication in each analysis (i.e., PSM for statins used aspirin use as a covariate while PSM for aspirin used statins use as a covariate). The caliper distance of the propensity score matching was determined by 0.2 of the standard deviation of the logit transformation score.27 To determine the robustness of the primary analysis, we performed a sensitivity analysis using all participants. In addition, we divided the patients into 3 groups to investigate the long-term effectiveness of the specific medications as follows: (1) continuous use, (2) never use, and (3) status change, which means those who reported receiving medication in phase 1 but did not in phase 2, or vice versa. We also investigated whether there are differences in the effects on muscles based on type of statins. p values less than 0.05 were considered statistically significant and all tests were 2-sided.
Results
Characteristics and Demographics of Study Participants
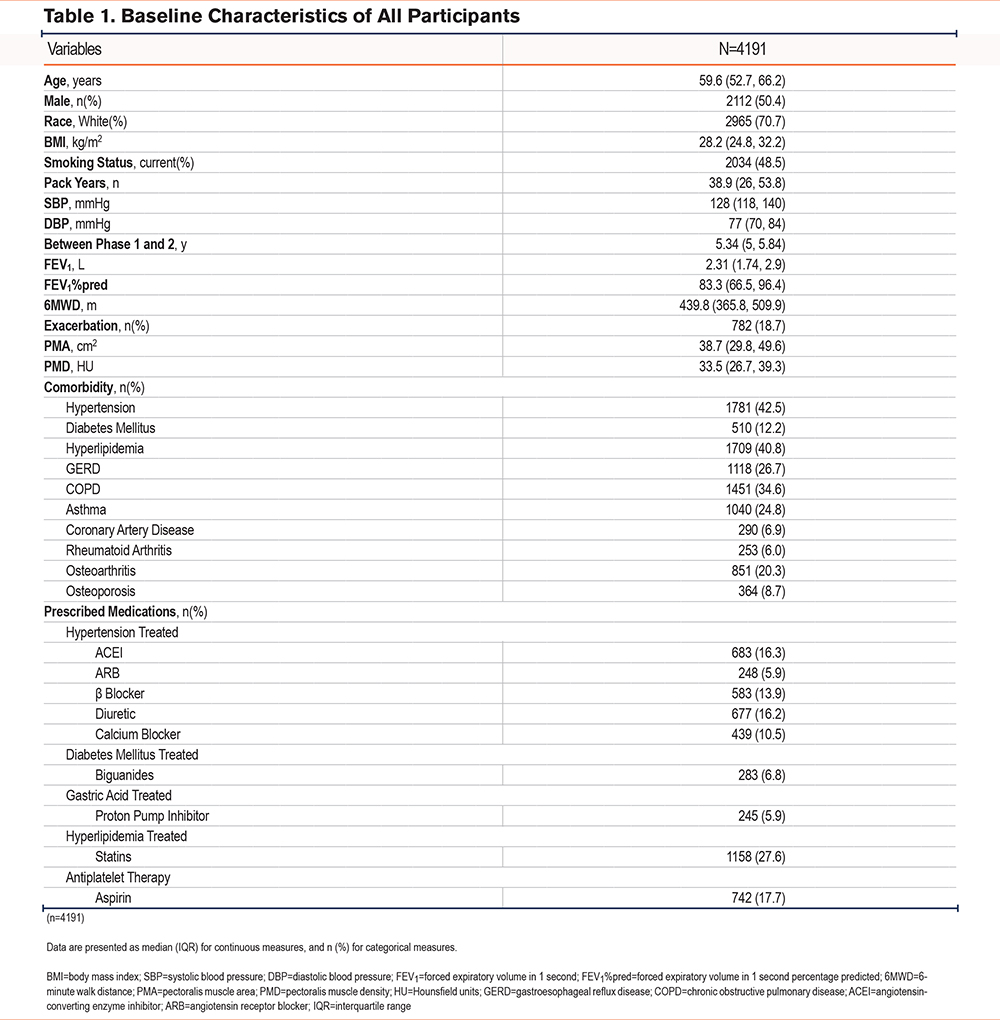
The selection of the study individuals is diagramed in Figure 1 with the baseline characteristics of the study participants shown in Table 1. The median age of all individuals was 59.6 years, and 50.4% were male. The median PMA and PMD were 38.7cm2 and 33.5HU, respectively, at their first visit. Comorbidities included hypertension which was reported by 42.5%, hyperlipidemia by 40.8% of participants, COPD by 34.6%, diabetes mellitus by 12.2%, and coronary artery disease by 6.9%. Regarding prescribed medications, antihypertensive use was reported by 42.5% of participants, antihyperlipidemia agents for 29.4%, antiplatelet agents for 19.2%, and antidiabetes mellitus agents for 8.4%
Regression Analyses for Delta-PMA With Prescribed Medications
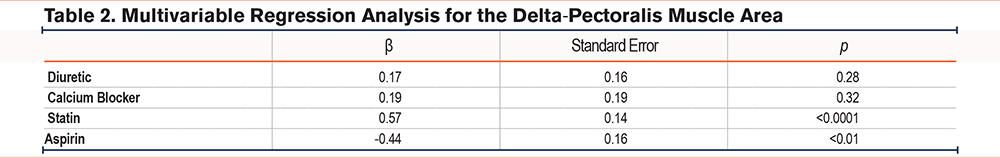
Over the 5-year period in the complete population of current and former smokers with longitudinal chest CTs, the median delta-PMA was -2.23cm2 (IQR: -6.52, 1.54) and the median delta-PMD was -3.22 HU (IQR: -8.44, 1.82). Bivariate regression analysis indicated statin use was associated with a lesser decrease in PMA (β=0.018, standard error [SE]=0.005, p<0.001) in comparison with nonusers. When adjustments were made for all medications with p-value <0.2 from the bivariate analyses (calcium channel blockers, diuretics, aspirin, and statins), aspirin use was associated with loss of PMA (delta-PMA: β=-0.44, SE=0.16, p<0.01) while statin use was robustly associated with less loss of PMA (delta-PMA: β=0.57, SE=0.14, p<0.0001) (Table 2). In the same analysis with PMD, statin use was associated with less loss of PMD (delta-PMD: β=0.40, SE=0.15, p<0.01) while aspirin use had no association with delta-PMD (β=0.003, SE=0.005, p=0.546).
Propensity Score Matching Analysis for Statins and Aspirin
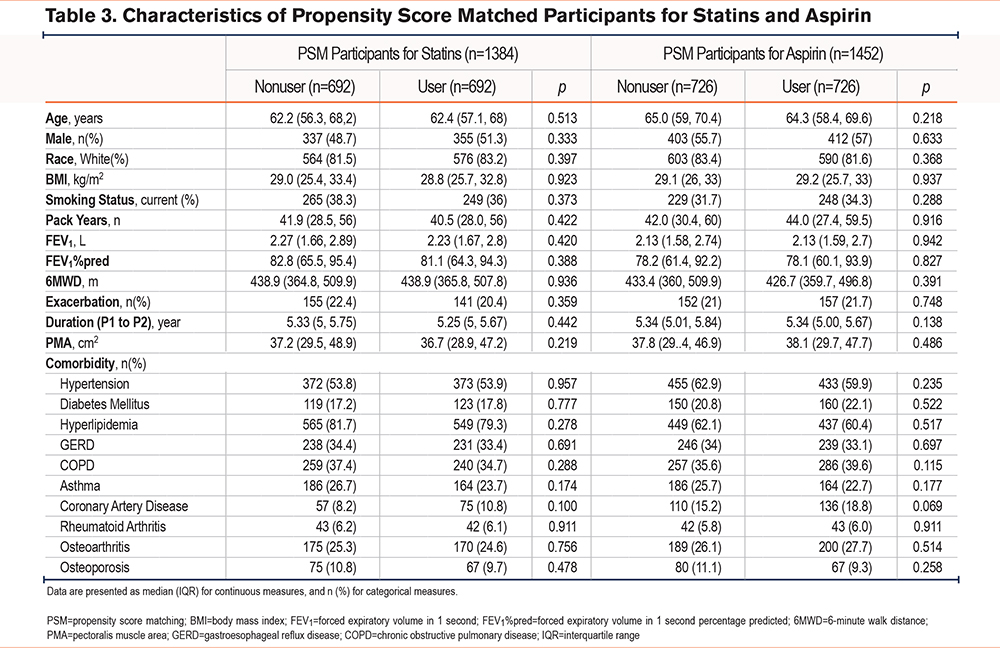
Of the 4191 participants included in this study, 1158 were statin users, and 742 were aspirin users. After 1:1 PSM, 692 pairs of participants for statin users and nonusers, and 726 pairs for aspirin users and nonusers were included, and the characteristics of PSM individuals with or without statin use, and aspirin use are shown in Table 3. As expected, PSM participants did not differ based on age, gender, BMI, smoking status, pack years, FEV1%pred, and comorbidities. There were no significant differences in COPD prevalence or in the number of patients using inhaled COPD treatments between the matched groups (statin versus nonstatin (38.0% versus 36.0%, p=0.737), and aspirin versus nonaspirin (42.7% versus 38.6%, p=0.183). Among individuals who reported receiving statins, 49.6% were prescribed simvastatin, 27.9% atorvastatin, 7.5% pravastatin, 7.8% rosuvastatin, and 7.1% lovastatin. In addition, one individual reported using multiple statins (0.1%).
Single Regression Analysis for Longitudinal Muscle Changes With Statin or Aspirin Use After Propensity Score Matching
Reported statin use was associated with a reduced loss of PMA compared to nonusers (median delta-PMA; -1.53 versus -2.54cm2, p=0.017) while aspirin users lost more PMA than that of non-users (median; -2.59 versus -1.92cm2, p=0.040) (Figure 2). When evaluating delta-PMD, statin use inhibited PMD worsening (median; -2.45 versus -3.71HU, p=0.004), demonstrating less muscle density loss in participants taking statins. On the other hand, no significant difference in delta-PMD was found between aspirin users and nonusers (median; -3.11 versus -2.89HU, p=0.566) (Supplementary e-Figure 1 in the online supplement).
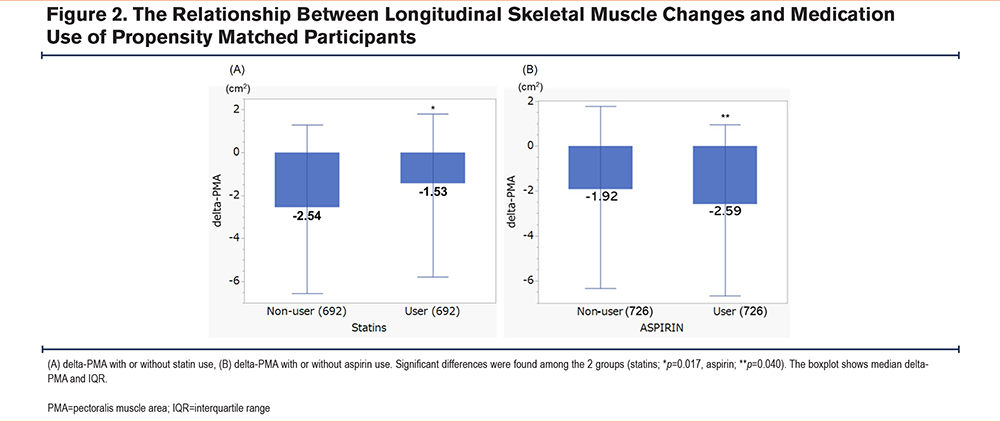
For participants taking statins, no significant differences in delta-PMA were observed on the basis of which statin a participant was taking (p=0.946), nor was there a significant difference between hydrophilic (pravastatin and rosuvastatin) and lipophilic (simvastatin, atorvastatin, and lovastatin) classes (p=0.728).
Effect of Persistent Statin Use
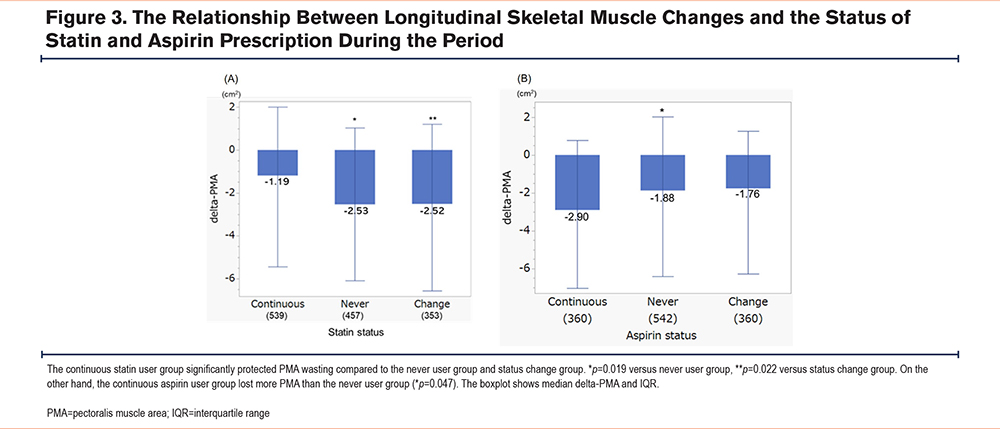
Participants were stratified into 3 groups based on their statin status during the study: continuous statin use (n=539), never statin use (n=457), and intermittent statin use (n=353). Figure 3 depicts the differences in delta-PMA among the 3 groups and the continuous use group showed less decrease in PMA than never use group and status change group (p=0.019 and p=0.022, respectively). On the other hand, for aspirin, continuous aspirin use (n=514) lost more PMA compared to only never use group (n=542) (p=0.047).
All Participants’ Analysis
In terms of all participants (n=4191), the statin user group (n=1158) had a lesser decrease in PMA and PMD compared to the nonuser group (n=3033) throughout the period (median delta-PMA; -1.73 versus -2.43cm2, p=0.0014, and median delta-PMD; -2.45 versus -3.55HU, p=<0.0001, respectively). In addition, after adjusting for the exact time between the 2 measurements, the yearly decline in PMA and PMD showed similar trends, with statin users having a smaller yearly decrease compared to nonusers (median delta-PMA; -0.44 versus -0.31cm², p=0.0019, and median delta-PMD; -0.63 versus -0.44HU, p=0.0001). When stratifying by smoking status, we found that statin users had a statistically significant beneficial effect in current smokers, with the statin user group showing significantly less muscle deterioration compared to nonusers (median delta-PMA; -1.82 versus -2.81cm², p=0.0132, and median delta-PMD; -1.56 versus -3.74HU, p<0.0001, respectively). However, in former smokers, there was no statistically significant difference in muscle outcomes between the statin user and nonuser groups (median delta-PMA; -1.71 versus -2.00cm², p=0.160, and median delta-PMD; -2.91 versus -3.15HU, p=0.354, respectively). As for aspirin, we did not observe any statistically significant differences in muscle deterioration between aspirin users and nonusers in either current or former smokers.
Discussion
In this study, we provided support for the concept that statin use among current and former smokers is associated with attenuated muscle loss in terms of mass (PMA) and quality (PMD).
Conversely, our study showed that aspirin use is associated with increased loss of pectoralis muscle area. As skeletal muscle loss impacts disease prognosis and quality of life in elderly patients, there is a need to determine whether our findings indicate medications currently being prescribed could be personalized to minimize skeletal muscle loss and/or reflect an underlying comorbid illness needing treatment.
Many studies have reported that statins have a negative side effect on skeletal muscle mass termed statin-associated myopathy (SAM).28-30 On the other hand, our findings indicate longitudinal statin may attenuate the loss skeletal muscle mass. There are several plausible reasons for the seemingly contradictory findings. First, patients who experience SAM typically discontinue statin use and in our study, the relationship between statin and less muscle loss was seen in participants with a history of statin use of at least 5 years. SAM occurs in approximately 10%–25% of patients receiving statin therapy,31 and accounts for approximately 90% of discontinuation cases.32 Second, the pleiotropic effects of statins can be considered. Statins have anti-inflammatory, antithrombotic, and immunomodulatory properties, reducing inflammatory mediators induced by tobacco exposure33 such as C-reactive protein, tumor necrosis factor-α, and interleukin-6. Given that inflammation significantly influences muscle mass and function, it offers a basis for suggesting a direct impact of statins on muscle loss in smokers.34 Smokers including COPD have a higher risk of cardiovascular diseases and diabetes, and statins can prevent events from cardiovascular diseases, improve lung function, and reduce the risk of acute exacerbations.35,36 As a result, statins probably can prevent overall health deterioration, resulting in the preservation of muscle proteins. Finally, skeletal muscles attenuation on CT scans have suggested the lipid infiltration of the muscle and is associated with changes in the metabolic health of skeletal muscles. The increase in muscle density due to statins may reflect differences in lipid metabolism, and as a result, it could be considered that the muscles of patients taking statins appear to be hypertrophic.37 In addition, as we described above, we demonstrated that the patients who took statins continuously for 5 years showed the less muscle loss in comparison with noncontinuous or intermittent statin users, which may suggest the importance of persistent treatment. However, it is possible that other unaccounted confounders explain the relationship between statin use and decreased pectoralis muscle loss. Additional work is needed, including prospective studies, but if confirmed, these findings would provide new insights into the role of statins for smokers and their potential importance independent of other indications for their use.
Interestingly, we found an opposite effect for aspirin. Aspirin is a well-known cyclooxygenase (COX) inhibitor that is commonly used as analgesic, antipyretic, anti-inflammatory, and cardioprotective medication. Prostaglandin E2 (PGE2), an inflammatory regulator whose synthesis is catalyzed by cyclooxygenases, has been associated with muscle regeneration, and its synthesis is induced by muscle damage.38-40 Aspirin, even at a low dose, reduces PGE2/COX pathway activity in resting human skeletal muscle.41,42 Thus, aspirin may hinder muscle regeneration and potentially lead to muscle degradation by inhibiting the synthesis of PGE2. This could explain why aspirin affects muscle area (PMA) but not muscle density (PMD). Since PMA reflects the size of the muscle, the inhibition of muscle regeneration through the suppression of PGE2 could lead to a reduction in muscle mass over time. On the other hand, PMD reflects muscle quality, including aspects like fat infiltration and structural integrity, which may be less affected by the PGE2 pathway. Therefore, while aspirin may impair the regenerative processes that influence muscle size, it might not significantly alter the underlying muscle quality. This distinction highlights the differential effects of aspirin on muscle mass and quality, which warrants further investigation.
This study has several limitations. First, medication use was self-reported, and dosage information, particularly for aspirin, was not available, limiting our ability to assess dose-dependent effects. Second, adherence to medication and reasons for prescription changes were not captured, which could influence the results. Third, the absence of a nonsmoking control group prevents us from distinguishing muscle loss due to smoking from age-related decline. Additionally, the lack of data on physical activity, particularly in participants with respiratory or cardiovascular conditions, may confound the association between medications and muscle changes. Furthermore, while our study used forced spirometry data, including measures such as CT-derived emphysema scores could provide a more comprehensive understanding of the relationship between emphysema and muscle mass loss. Finally, although propensity score matching was used to reduce confounding, unmeasured factors related to medication indications may still have influenced the findings. Future research should address these limitations, including incorporating nonsmoking controls, physical activity measures, and detailed medication dosage data.
Conclusion
In conclusion, our study illuminated the contrasting effects of statin and aspirin use on longitudinal pectoralis muscle area in ever smokers. Statin use among current and former smokers appears to be associated with a reduction in muscle loss, both in terms of mass (PMA) and quality (PMD), potentially attributed to the pleiotropic effects of statins. Clarifying the impact of statins and aspirin on skeletal muscle mass in ever smokers is crucial for tailoring medication prescriptions to minimize muscle loss and improve overall health outcomes in this population. Although additional research is needed, these findings raise new questions regarding the role and importance of statin and aspirin use in smokers.
Acknowledgment
Author contributions: TS, RSJE, SYA, GRW, and FNR, contributed towards conception and design of the study. TS, SYA, and FNR analyzed the data. NAE, VC, BC, and AAD provided critical interpretation of the data and contributed towards manuscript preparation. JC and MNM reviewed the statistics and provided critical insights. TS wrote the manuscript. All authors have approved the final version of the manuscript.
We thank all COPDGene investigators for allowing us to perform the current analysis.
Declaration of Interest
AAD reports grants from the National Heart, Lung, and Blood Institute(NHLBI) (R01-HL149861, R01-HL164824), USPT patent pending “NOVEL ASSAYS TO DETECT RESPIRATORY DISEASES AND DISORDERS.” JC reports funding support from the University of Alabama at Birmingham, Heersink School of Medicine, Department of Medicine. BC reports grants from the National Institutes of Health (NIH) and the American Lung Association, and consulting fees from Quantitative Imaging Solutions. MNM reports grant from the NIH. SYA reports grant support from the NHLBI (K08-HL145118) and the Pulmonary Fibrosis Foundation, consultant fees from Verona Pharmaceuticals and Vertex Pharmaceuticals, and ownership from Quantitative Imaging Solutions, a company specializing in imaging analytics in the lung cancer space. RSJE reports grants from the NHLBI, contracts with Lung Biotechnology and Insmed, a sponsored research agreement with Boehringer Ingelheim, consulting fees from Leuko Labs and Mount Sinai, and a patent pending in the area of lung cancer risk assessment using machine learning technology. He is cofounder and stockholder of Quantitative Imaging Solutions, and a board member of Fundación MVision. GRW has received institutional funding from the NIH, the Department of Defense and Boehringer-Ingelheim, and has received consultant fees from Vertex, Janssen Pharmaceuticals, Pieris Therapeutics, Intellia Therapeutics, and Regeneron. He is a cofounder and equity share holder in Quantitative Imaging Solutions. His spouse works for Biogen. The other authors have no conflicts of interest to declare.