Running Head: Triple Inhaler Therapy in COPD
Funding Support: None
Date of Acceptance: December 9, 2024 | Publication Online Date: December 13, 2024
Abbreviations: aOR=adjusted odds ratio; CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; DPI=dry powder inhaler; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; ICS=inhaled corticosteroid; IQR=interquartile range; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; MARS-A=Medication Adherence Report Scale for Asthma; MDI=metered-dose inhaler; MITT=multiple inhaler triple therapy; mMRC=modified Medical Research Council; OR=odds ratio; QMH=Queen Mary Hospital; REDCap=Research Electronic Data Capture software application; SD=standard deviation; SITT=single inhaler triple therapy
Citation: Kwok WC, Ma TF, Tsui CK, Ho JCM, Tam TCC. Prospective randomized study on switching triple inhaler therapy in COPD from multiple inhaler devices to a single inhaler device in a Chinese population. Chronic Obstr Pulm Dis. 2025; 12(1): 52-60. doi: http://doi.org/10.15326/jcopdf.2024.0519
Online Supplemental Material: Read Online Supplemental Material (284KB)
Background
Among patients with chronic obstructive pulmonary disease (COPD) who have exacerbations with a high blood eosinophil count, triple therapy consisting of an inhaled corticosteroid (ICS) plus a long-acting beta2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) is recommended in the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines with benefits in terms of mortality and moderate-to-severe exacerbations.1-10 Triple therapy can be administered by single inhaler triple therapy (SITT) or multiple inhaler triple therapy (MITT). In a recently published Delphi consensus, there was agreement among experts regarding the appropriate clinical use and benefits of triple therapy in COPD, including its mortality benefits, the comparable pneumonia risk between SITT and MITT, the preference of SITT for patients with high eosinophil counts and the exacerbation risk reduction and health care cost benefits of early initiation of SITT for a post- exacerbation−related hospitalization (<30 days).11 When compared with MITT, SITT has been shown to be associated with improved treatment persistence and adherence, as well as a reduction in the risk of moderate-to-severe exacerbations, severe exacerbations, and mortality.12-14A real-world observational study in the United Kingdom showed that patients with SITT had improved lung function and a higher proportion of COPD Assessment Test (CAT) improvement at 24 weeks compared with the MITT group.15
There has been a lack of research on the benefits of SITT over MITT in the Chinese population, with a recently published study focused on the benefits of SITT in newly diagnosed cases of COPD.12 Whether the same benefits persist in patients who are already on MITT has not been studied in the Chinese population. As such, we conducted this prospective study on treatment adherence, patient satisfaction, critical inhaler errors, and symptom control for Chinese patients with COPD who were already on MITT.
Methods
This open-label, double-arm clinical trial was conducted at Queen Mary Hospital (QMH), Hong Kong. All Chinese patients with COPD who were already on triple therapy (LABA/LAMA/ICS) via MITT and who were followed at QMH from January 25, 2022, to March 14, 2023, were included. The diagnosis of COPD was confirmed by spirometry demonstrating postbronchodilator airflow limitation with a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio < 0.7. Exclusion criteria included co-existing asthma, bronchiectasis, and asthma/COPD overlap. The study was approved by the institutional review board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (approval number UW19-712). All patients consented to join the study; written informed consent was obtained. The study was conducted in accordance with the Declaration of Helsinki. The recruited patients had their history taken, a physical examination, spirometry, and their blood taken at the time of recruitment. At the screening visit, detailed history taking, physical examination, as well as baseline assessments (body mass index, a complete blood count, a liver and renal function test, a chest X-ray, spirometry, the modified Medical Research Council [mMRC] Dyspnea Scale, and the Medication Adherence Report Scale for Asthma [MARS-A]) were collected. Randomization was performed by the Research Electronic Data Capture (REDCap)16 web application and stratified by age, gender, and smoking status. While the MARS-A was initially developed for asthma, it has also been validated in patients with COPD.17 The number of inhaler critical errors was checked by asking the patient to demonstrate their inhaler techniques. Inhaler technique was checked at baseline at the time of recruitment. Patients were randomized to continue triple therapy with the MITT they were using (MITT group) or were switched to triple therapy with SITT via the Ellipta® device (SITT group). For patients who were prescribed a metered-dose inhaler (MDI), a spacer was also given to them for free from the pharmacy and they were taught to use the MDI with the spacer. They were taught by pharmacists on the inhaler technique again after randomization and followed up at 4 and 12 weeks . At the face-to-face inhaler teaching session, the patients were taught by the pharmacists on the proper inhaler technique including a demonstration with a placebo device. Afterward, the patients were also asked to demonstrate to the pharmacist how to use the inhaler. The patients were only prescribed an inhaler if their inhaler technique assessment by a pharmacist was deemed satisfactory. Patients were assessed on the mMRC dyspnea scale, the MARS-A score, their inhaler satisfaction by the ease-of-use questionnaire,18 and their number of critical inhaler errors on follow-up visits.18-20
Critical errors were defined as errors that seriously compromised drug delivery to the lungs and included dose preparation and dose delivery errors. The summary of the critical errors for each type of inhaler device, which was adopted by prior studies, is included in Supplementary Table 1 in the online supplement.
The hypothesis to be tested included whether the switch from MITT to SITT in patients with COPD who were on MITT before study recruitment would lead to changes in their mMRC dyspnea scale, their MARS-A score, their satisfaction score via the ease-of-use questionnaire, and their number of critical errors. The outcomes were assessed as continuous variables by the absolute changes in these scores and scales. The outcomes were also assessed as the percentage of patients who had improvement in these parameters. The assessments were done at 4 and 12 weeks after recruitment.
The primary outcome is the number of critical errors between the 2 groups – the SITT group and the MITT group. The secondary outcome includes the mMRC dyspnea scale scores, the MARS-A scores, and satisfaction scores via the ease-of-use questionnaire between the 2 groups.
A sample size of 70, with 35 in each group, was estimated to be needed based on the primary outcome. The calculation of sample size is included in Appendix 1 in the online supplement.
Statistical Analysis
The demographic and clinical data are described in actual frequency, mean±SD or median (interquartile range [IQR]). Baseline demographic and clinical data were compared between the 2 groups (SITT or MITT) with independent t-tests or nonparametric tests where appropriate. For the primary endpoint of interest (treatment compliance, satisfaction of the inhaler, mMRC dyspnea scale, and the number of inhaler errors), the baseline and measurements at 4 and 12 weeks for paired samples were tested using a nonparametric Wilcoxon signed-rank test. To identify the percentage of patients in the SITT and MITT groups with improvement in the study parameters, a univariate logistic regression model was performed. A multiple logistic regression model was used to assess potential confounders that included age, baseline mMRC dyspnea scale, and baseline FEV1. The factors above were identified as potential founders, based on the literature,21,22 for potential impact on the effect due to inhaler technique and mMRC dyspnea scale on follow-up visits. The effect due to inhaler and mMRC dyspnea scale and confounding variables (age and FEV1) is illustrated as a hypothetical directed acyclic graph in the supplementary Figure 1 in the online supplement. The difference in causal effect in the odds ratio (OR) was also estimated. Linear regression was used to estimate the association, the mean number of the study parameters, and the treatment groups. Bonferroni correction was employed for multiple comparisons. The statistical significance was determined at the level of p=0.05 at the 2-sided test. All statistical analyses were done using the SPSS statistical package, version 28 (IBM; Armonk, New York), and R Statistical Software (version 4.2.2, R Foundation; Vienna, Austria).
Results
A total of 73 Chinese patients with COPD managed in Queen Mary Hospital were included. Three were excluded as they dropped out of the study, with 33 in the MITT group and 37 in the SITT group.
Baseline Characteristics
The mean age was 72.3±8.4 years. There were more male patients (92.9%). The mean FEV1 was 1.43±0.61 L (64.4±22.5%). The mean mMRC score was 1.94±0.81. The mean MARS-A score was 45.7±6.6. The results are summarized in Table 1.
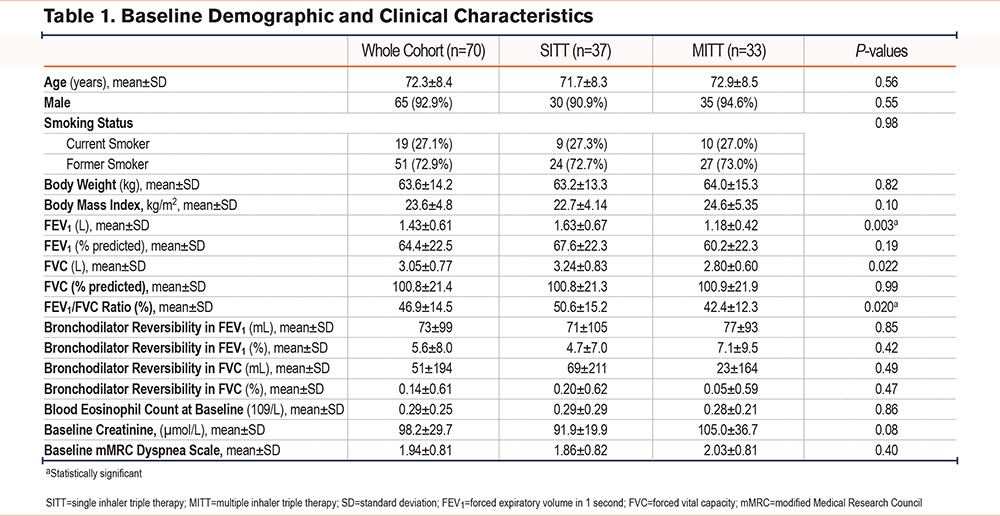
Number of Critical Inhaler Errors
The mean number of critical errors per inhaler at baseline assessment was 1.5±1.6 in the SITT group and 2.2±2.0 in the MITT group, p=0.10. The mean number of critical errors per inhaler was significantly lower in the SITT group at the first and second visits. The mean number of critical errors was 0.4±1.0 in the SITT group and 1.1±1.8 in the MITT group, p=0.038 at the first visit. The mean number of critical errors was 0.2±0.6 in the SITT group and 0.8±1.1 in the MITT group, p=0.007 at the second visit. The result was a statistically significant difference for the mean number of critical errors per inhaler at the second visit adjusted for age, gender, FEV1, FEV1/FVC ratio, and baseline mMRC dyspnea scale, with a p-value of 0.029 (Figure 1, Table 2).
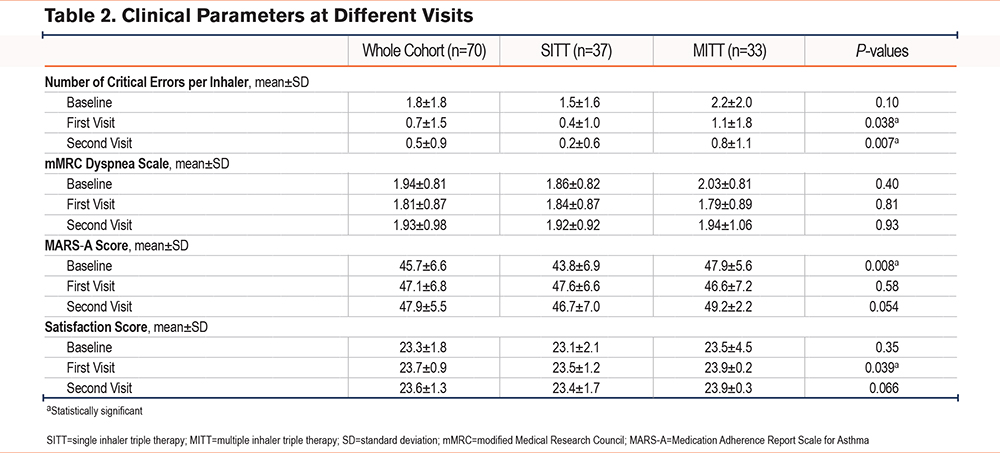
mMRC Dyspnea Scale
The mean baseline mMRC dyspnea scale was 1.86±0.82 and 2.03±0.81 in the SITT and the MITT group respectively, p=0.40. The mean mMRC dyspnea scale scores were 1.84±0.87 and 1.79±0.89 in the SITT and the MITT group, respectively, at the first visit, p=81 and 1.92±0.92 and 1.94±1.06 in the SITT and the MITT group at the second visit, p=0.93. There was no statistically significant difference in the mean mMRC dyspnea scale scores in the first visit at 4 weeks and the second visit at 12 weeks (Table 2). The mean change in the mMRC dyspnea scale scores from baseline to the first visit was -0.03±0.65 in the SITT group and -0.24±1.00 in the MITT group, p-value=0.28 in univariate analysis and 0.35 in multivariate analysis adjusted for age, gender, FEV1, FEV1/FVC ratio and baseline mMRC dyspnea scale score. The mean change in mMRC dyspnea scores from baseline to the second visit was +0.05±0.71 in the SITT group and -0.09±1.21 in the MITT group, p-value=0.28 in univariate analysis and 0.71 in multivariate analysis adjusted for age, gender, FEV1, FEV1/FVC ratio, and baseline mMRC dyspnea score.
MARS-A Score
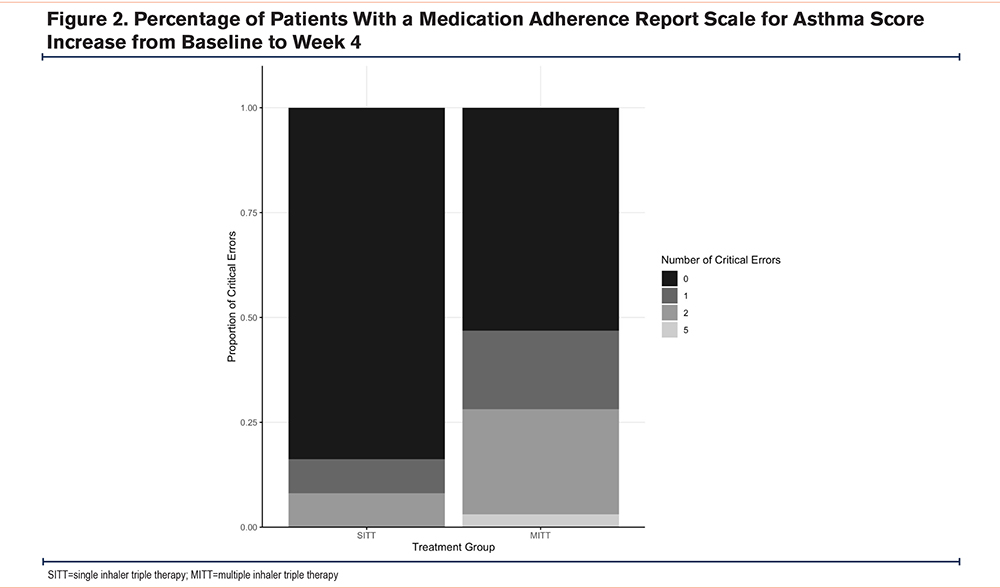
The mean baseline MARS-A scores were 43.8±6.9 and 47.9±5.6 in the SITT group and the MITT group respectively, which is significantly higher in the MITT group, p=0.008. There was no statistically significant difference in the mean MARS-A scores in the first visit and second visit, with the mean MARS-A score being 47.6±6.6 and 46.6±7.2 in the SITT group and the MITT group respectively, at the first visit, p=0.58; and 46.7±7.0 and 49.2±2.2 in the SITT group and the MITT group at the second visit, p=0.054. The mean change in the MARS-A scores from baseline to first visit was +3.76±7.48 in the SITT group and -1.27±7.76 in the MITT group, p-value=0.008. The result was statistically significantly different after adjusting for age, gender, FEV1, FEV1/FVC ratio, and baseline mMRC dyspnea score, with a p-value of 0.008. A total of 22 (59.5%) and 8 (24.2%) of the patients in the SITT and the MITT groups had an increase in MARS-A scores from baseline to the first visit respectively, p=0.003 (Figure 2). The OR for an increase in the MARS-A score was 4.58 (95% confidence interval [CI] =1.63–12.86, p=0.004). The adjusted OR (aOR) was 6.23 (95% CI=1.63–23.77, p=0.007) suggesting that the SITT group had higher odds of having an increase in MARS-A score from baseline to the first visit. The risk difference is 35.22% (95% CI=13.68%−56.76%), p=0.004. The mean change in MARS-A scores from baseline to the second visit was +2.89±9.66 in the SITT group and +1.30 ±6.68 in the MITT group, p-value 0.42 (Table 2). By directed acyclic graph, the estimated direct effect due to inhaler device (MITT or SITT) dominates the total effect on the response, which is similar to the finding in the univariate and multivariate analysis (Supplementary Table 2 in the online supplement).
Satisfaction Score as in Ease-of-Use Questionnaire
The mean satisfaction score was 23.1±2.1 in the SITT group and 23.5±4.5 in the MITT group, p=0.34. The mean change in satisfaction score from baseline to first visit was +0.38±2.43 in the SITT group and +0.42±1.48 in the MITT group, p-value=0.92 in univariate analysis and 0.66 in multivariate analysis adjusted for age, gender, FEV1, FEV1/FVC ratio, and baseline mMRC dyspnea score. The mean change in satisfaction score from baseline to the second visit was +0.27±2.59 in the SITT group and +0.42±1.46 in the MITT group, respectively, p-value=0.76 in univariate analysis and 0.63 in multivariate analysis adjusted for age, gender, FEV1, FEV1/FVC ratio, and baseline mMRC dyspnea score (Table 2).
Discussion
In this study, we demonstrated that switching from MITT to SITT may have the benefits of having lower critical inhaler error numbers and higher MARS-A scores, as early as 4 weeks. The potential benefits could persist for 12 weeks after the switch. The results of our study are consistent with prior studies that suggest the potential benefits of SITT. Switching triple therapy from MITT to SITT might have the benefit of improving adherence to triple therapy with lower critical errors.
While both SITT and MITT are available for COPD, determining whether switching from MITT to SITT is beneficial remains a question clinicians frequently ask. Both clinicians and patients may be hesitant to change the current inhaler therapy if it is effective and well tolerated. In our study, the possible benefits of switching triple therapy from MITT to SITT in patients with COPD were suggested as improving adherence and reducing the number of critical errors. Our observation may provide evidence to support this change in inhaler therapy and clinicians may consider the switch from MITT to SITT for patients with Group E COPD, especially if there are concerns about medication adherence and critical errors from existing inhalers.
The development and introduction of SITT is a breakthrough in COPD inhaler pharmacotherapy. Once daily SITT can bring about convenience to the patients. Yet, SITT is currently available as a dry powder inhaler (DPI) (Ellipta® and Breezhaler®) and a metered-dose inhaler (MDI) only. This has both pros and cons from patients’ and clinicians’ perspectives. The concern about the inspiratory flow and suitability of DPI is always a concern.23 The main drawback of an MDI is the need for hand-mouth coordination.24 These concerns are particularly relevant in places where soft mist inhalers are the most commonly used inhaler device for LABA/LAMA combinations, as the technique for using a DPI is completely different from how a soft mist inhaler is used. It is important to assess if the switch from MITT to SITT is beneficial among patients who have been on MITT, especially for preparations consisting of soft mist inhalers, which is not available as a SITT.
Our study suggested that switching from MITT to SITT may lead to an improvement in critical inhaler error numbers and a higher MARS-A score. The results may reassure clinicians when they have a choice to switch to SITT from MITT for their patients with COPD. The patients may benefit from the switch in inhaler device by having better inhaler adherence and fewer critical inhaler errors. A higher medication adherence is important as medication compliance and adherence is the cornerstone of chronic disease management. However, we did not observe any significant difference regarding the change in the mMRC dyspnea scale in the 2 groups despite better adherence as measured by the MARS-A score and fewer critical errors in the SITT group. This could be because all the patients recruited had Group E COPD and required a LABA/LAMA/ICS combination before the recruitment. The change from MITT to SITT without other major changes in pharmacotherapy may not be able to bring about significant changes in the mMRC dyspnea scale as the patients in both treatment groups were receiving a LABA/LAMA/ICS combination which has similar clinical benefits for all.
We also did not observe any significant difference in the satisfaction score in this study. While SITT was associated with better adherence and fewer critical errors, satisfaction with the inhaler device, which is a more subjective measure, may not be better when MITT is replaced by SITT. Whether patients are more satisfied with a particular inhaler device will depend on both patient factors as well as device factors. As a DPI was the SITT in this study, some patients may not be more satisfied when switched from MITT to SITT as they were started on a new inhaler device, and DPI may not be the most preferred inhaler for them.
Another point to note in this study is that patients who switched from MITT to SITT had worse lung function as measured by FEV1. Despite having more severe COPD, they were still able to use SITT well with benefits seen as early as 4 weeks. This suggests that having more severe COPD should not be considered as an absolute contraindication to SITT with Ellipta® as long as they have adequate inspiratory flow rate and proper inhaler technique.
In our study, despite the fact that the patients were all taught by pharmacists on inhaler techniques, the patients still had critical errors upon follow-up. Adopting a standardized training model involving verbal instructions and device demonstrations by pharmacists may improve patients' ability to use the inhalers. Furthermore, assessing patients' level of understanding of the disease is also important as it has been reported that patients' acceptance of the disease process and recommended treatment, knowledge about, and faith in the treatment are factors affecting medication adherence in COPD.25
We also noted that all the patients in this study had fewer critical errors in the first and second visits when compared to baseline, while the changes were more prominent in the SITT group than the MITT group. In our study, all patients received the inhaler technique education and were checked by a pharmacist after recruitment. This may have helped to improve the inhaler techniques of the patients in both groups with subsequent improvement in the number of critical errors. This finding illustrated the importance of inhaler technique education and checking, a cornerstone to successful inhaler therapy. In our study, the MARS-A scale was used to measure treatment adherence, which has been used in other studies and validated in COPD. However, in the current era, a better way to measure patients’ compliance with medication including inhaler use may be to include integrating electronic monitoring devices into the treatment regimen. While the MARS-A scale was shown to have good internal validity when measured among patients with COPD or asthma, it was weakly correlated with objectively measured medication adherence, with low levels of sensitivity and specificity. Using electronic monitors, when available, is a better approach to monitoring treatment adherence.
Our study has several limitations to address. First, the study was conducted in one tertiary center. The relatively small sample size with a short duration of follow-up may not have allowed for the detection of small differences among the 2 groups, especially for the longer-term outcome. A larger-scale study among the Chinese population will allow for a better assessment of the outcomes. Despite this, the results from our study are consistent with previous reports in the literature. Peak inspiratory flow rate was not measured in this study, as the main outcomes of interest were treatment adherence, satisfaction, and critical error rates. Yet, peak inspiratory flow rate is considered one of the key factors in selecting an inhaled medication delivery system for patients, particularly with DPIs. A separate study focusing on peak inspiratory flow rate among patients with severe COPD is worth conducting. Furthermore, all the patients recruited including the MITT and SITT groups, had been on triple therapy for COPD. Whether the same benefits are seen among patients on other regimes such as single or dual-bronchodilators without an ICS is not confirmed in this study. A separate study is needed to assess the benefits in these patients who are not indicated for triple therapy. Also, different MITTs were used as a baseline for the included patients. Given the small scale of this study, we were not able to precisely assess the clinical benefits for each individual combination with MITT.
Conclusion
Switching from MITT to SITT in Chinese COPD patients may have the benefits of having less critical inhaler errors and higher MARS-A scores. Clinicians should consider switching from MITT to SITT in appropriate settings.
Acknowledgements
Author contributions: WCK was involved with the study concept and design, analysis and interpretation of data, acquisition of data, drafting of the manuscript, and approval of the final version of the manuscript. JCMH, TFM, and CKT were involved with critical revision of the manuscript for important intellectual content and approval of the final version of the manuscript. TCCT was involved with the study concept and design, drafting of manuscript, critical revision of the manuscript for important intellectual content, study supervision, and approval of the final version of the manuscript.
Data Sharing statement: All available data is presented in the manuscript and no additional data will be provided. Data is not available to be shared.
Declaration of Interests
The authors declare no conflict of interests.