Running Head: Iron Deficiency and Hospitalization Risk in COPD
Funding: This work was supported by National Heart, Lung, and Blood Institute grants T32HL007534 (YK), F32HL170557 (YK), and K23HL153778 (AB). It was also supported by Johns Hopkins inHealth Precision Medicine Initiative grant funding and Highmark Health/Allegheny Health Network collaboration research funding.
Date of Acceptance: December 9, 2024 | Publication Online Date: December 16, 2024
Abbreviations: BMI=body mass index; CBC=complete blood count; CHF=congestive heart failure; CI=confidence interval; CKD=chronic kidney disease; COPD=chronic obstructive pulmonary disease; DM= diabetes mellitus; EHR=electronic health record; eGFR=estimated glomerular filtration rate; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GLI=Global Lung Function Initiative; ICD-10=International Classification of Diseases, Tenth Revision; IQR=interquartile range; JHHS=Johns Hopkins Health System; OR=odds ratio; PFT=pulmonary function test; PMCOE=Precision Medicine Center of Excellence; TIBC=total iron binding capacity; TSAT=transferrin saturation
Citation: Kunitomo Y, Putcha N, Fawzy A, et al. Iron deficiency and all-cause hospitalization risk in a clinical cohort of COPD. Chronic Obstr Pulm Dis. 2025; 12(1): 72-81. doi: http://doi.org/10.15326/jcopdf.2024.0550
Online Supplemental Material: Read Online Supplemental Material (464KB)
Note: Part of this work was presented at the American Thoracic Society 2024 International Conference in San Diego, California
Introduction
Comorbid diseases and multimorbidity are important determinants of clinical outcomes among individuals with COPD.1,2 Iron deficiency is a highly prevalent condition affecting an estimated 3.7 billion individuals globally, and has been a comorbidity of interest for chronic diseases such as congestive heart failure (CHF) and chronic kidney disease (CKD) as a potential treatable trait.3-5 Investigations of iron deficiency in COPD, however, remain nascent with limited data on prevalence or associations with outcomes.
Iron is an essential element for cellular bioenergetics, oxygen transport, and oxidative metabolism.6 In the lungs, iron homeostasis is also relevant to immune defense against infection and the physiologic response of hypoxic vasoconstriction.7-9 Iron deficiency may impair these vital functions independent of the presence of anemia, leading to increased morbidity in COPD which itself leaves individuals prone to infection, hypoxia, and altered bioenergetics.10 Additionally, COPD is frequently complicated by CHF, where iron deficiency is associated with worse outcomes.11 Therefore, individuals with COPD may be particularly sensitive to being iron deficient.
Small observational studies in the COPD population have reported a prevalence of iron deficiency of 10%−40%, including potential associations with COPD exacerbations, exercise tolerance, and pulmonary hypertension.12-16 While informative, the majority of these studies did not distinguish between states of iron deficiency and iron deficiency anemia, making it difficult to isolate the impact of iron deficiency, independent of anemia, on outcomes. Second, iron deficiency was defined primarily using serum ferritin levels, which are now increasingly recognized to have poor sensitivity among individuals with chronic diseases.17,18 Finally, there are differences in iron metabolism between men and women which have not been previously addressed.19
This study aimed to elucidate the prevalence of iron deficiency with and without anemia in a clinical COPD cohort and to understand whether it is associated with the risk of hospitalization among patients with COPD. Further, iron deficiency was defined using transferrin saturation (TSAT) to address limitations with defining iron deficiency using ferritin. We hypothesized that there would be a high prevalence of iron deficiency among individuals with COPD, and iron deficiency would be associated with a higher risk of hospitalization, regardless of anemia status. Understanding the impact of iron deficiency in COPD and its relationship to anemia status may better inform the need for further study of this treatable comorbidity, as well as identify the COPD subpopulations that may be more vulnerable to the effects of systemic iron deficiency.
Methods
Study Population and Design
This was a retrospective cohort study using electronic health record (EHR) data from the Johns Hopkins COPD Precision Medicine Center of Excellence (PMCOE) data repository. The COPD PMCOE data registry is comprised of adults aged ≥40 years who were seen in the Johns Hopkins Healthcare System (JHHS) between July 2016 and January 2023 with COPD (based on diagnosis code or airflow obstruction by forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio of <0.7). Individuals with at least one outpatient iron profile measurement (serum iron level, total iron binding capacity [TIBC], transferrin, ferritin) between July 2016 and January 2022 and at least 1 year of subsequent follow-up time were included. The timing of the iron labs was considered time=0 with outcomes measured over the following 1-year period and administrative censoring after 1 year. Individuals were excluded if they had hematologic disorders known to affect iron profile measurements (myelodysplastic syndrome, hemophagocytic lymphohistiocytosis, polycythemia vera) identified by International Classification of Diseases, Tenth Revision (ICD-10) codes and if they had abnormally high iron levels (see definitions below), as it was thought to represent a different disease state. Istitutional review board approval was obtained from all JHHS sites with a waiver of consent as this study was deemed a minimal risk.
Exposure and Outcome Measurements
The first outpatient iron profile measurements after July 2016, including serum iron, transferrin, TIBC, and ferritin, available for each individual were used to determine iron status. TSAT was calculated from the iron level and TIBC ([iron µg/dL/TIBC]×100%). Individuals were categorized by TSAT using thresholds historically utilized in literature20 to define iron deficiency and overload: iron deficient TSAT ≤20%, normal 20%<TSAT≤50%, and high iron TSAT >50%. The complete blood count (CBC) obtained closest to the iron profile measurement was used to determine hemoglobin and anemia status, with anemia defined as hemoglobin <12g/dL for females and <13g/dL for males.21 The primary outcome was all-cause hospitalizations as a dichotomous variable (ever versus never) within the year after the initial iron profile measurement. All-cause hospitalizations were defined as any inpatient, observation, or emergency department encounter.
Clinical characteristics defined at the time of iron profile measurement included age, sex, self-reported race, height, weight, body mass index (BMI), and smoking status (ever versus never). We did not distinguish between ever smokers as current versus former and did not include previous smoking burden (pack years) given the inaccuracy of current smoking status in the EHR which is infrequently updated and the lack of a systematic way of recording pack-year history within the EHR. Comorbid conditions (CHF, diabetes mellitus [DM], CKD, liver disease) were abstracted as dichotomous variables (ever versus never) based on ICD-10 codes. The estimated glomerular filtration rate (eGFR) was used to categorize individuals as having eGFR≥60 (CKD stage 2 or lower) versus eGFR<60 (CKD stage 3 and higher) to reflect the nonlinear association between eGFR and anemia prevalence.22 Lung function was characterized by FEV1 percentage predicted from available pulmonary function tests (PFT). All PFTs done within the JHHS were conducted and interpreted using the European Respiratory Society/American Thoracic Society guidelines with z-scores and percentage predicted values calculated using the Global Lung Function Initiative (GLI) global reference equations.23,24 COPD hospitalizations were defined by admission ICD-10 code J44.1, which has been previously validated in this population.25 Medication history was obtained from medication orders and administration history encompassing inpatient and outpatient encounters from each individual. Prescription for iron supplementation was defined as a binary variable for oral and/or intravenous iron prescription during the first year of follow-up after iron profile measurement, which included both incident and prevalent users. All other medication history was determined as ever versus never use.
Statistical Analysis
Participant characteristics were compared across iron status (deficient versus normal) using t-tests and Chi-squared tests, as appropriate. Additionally, characteristics among iron-deficient participants were compared by anemia status (non-anemic versus anemic) and stratified by sex.
The main analysis assessed the association between TSAT and all-cause hospitalization over the 1-year follow-up period. TSAT was treated both continuously within the low to normal TSAT range of 0%−50%, and as a categorical variable (low and normal). The main model utilized logistic regression adjusting for age, BMI, race (White versus non-White), CHF, eGFR category (eGFR≥60 versus eGFR<60), and history of COPD hospitalization (in the year prior to iron measures), with an interaction term for anemia to test effect modification. The 2 comorbidities of CHF and CKD were included because of their known association with iron deficiency, as well as anemia for the latter, and the outcome of hospitalization risk.3,26 CHF was included as a binary variable (yes versus no) as discrete data from echocardiograms was not available from the EHR. All analyses were stratified by sex given known differences in iron homeostasis.19 A sensitivity analysis, limited to patients with spirometric airflow obstruction defined as FEV1/FVC <0.7 or less than the lower limit of normal, with the percentage predicted FEV1 as an additional covariate to account for COPD disease severity, was performed. All analyses were conducted using Stata 18.0 (StataCorp LLC; College Station, Texas) with significance for main effects27 set at a p-value of <0.05 and for interactions set as a p-value <0.1.
Results
Participant Characteristics and Distribution of Iron Measures
Of the 69,077 individuals in the COPD PMCOE registry, 6532 individuals had iron profiles available and were included in the final sample, (Supplemental Figure 1 in the online supplement) with an average age of 65.0 ± 11.9 years, 59% female, and 56% White. Fifty-two percent (n=3385) of the cohort were iron deficient (TSAT ≤20%), of whom 27% (n=919) were non-anemic. Patient characteristics by iron status are shown in Table 1. This study cohort had similar rates of all-cause and COPD hospitalization compared to the overall COPD PMCOE registry. Compared to individuals with normal iron, iron-deficient patients had, on average, a higher BMI, lower lung function, and a higher prevalence of chronic heart failure and diabetes. Iron supplementation was more prevalent in the iron-deficient group at 24.3% compared to 8.7% in the normal iron group. Prevalence of ever use of aspirin, other antiplatelet agents, and oral anticoagulation agents were similar across the 2 groups.
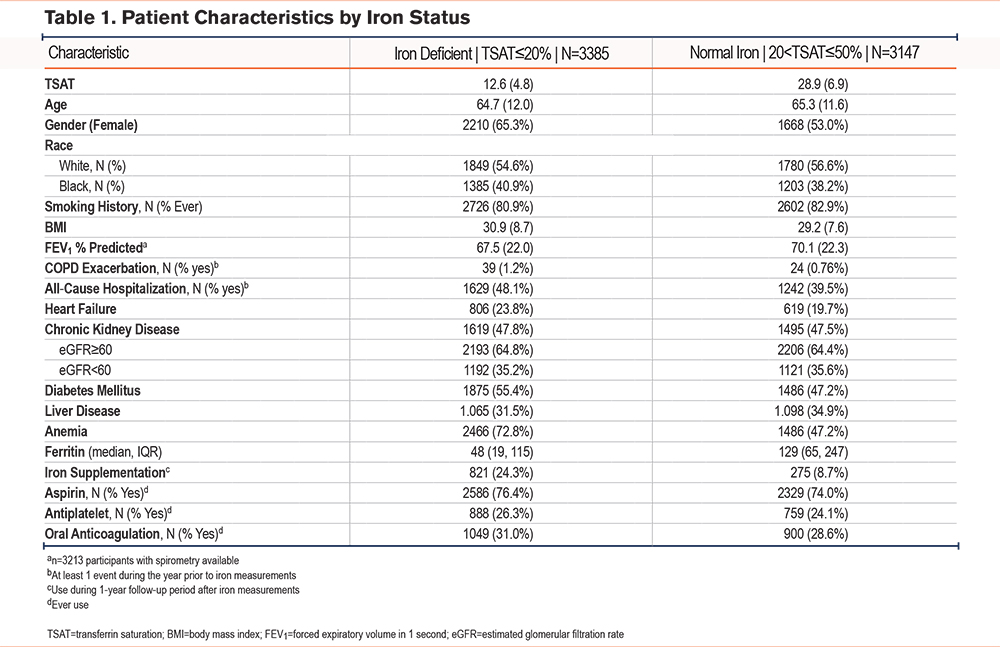
Iron deficiency was more prevalent among females at 57% compared to 44% among males. This trend held true regardless of anemia status (Figure 1). Mean TSAT among females was 19.1% ± 10, and among males was 22.4% ± 10. Median ferritin for females was 65 (interquartile range [IQR] 27, 141), and median for males was 117 (IQR 49, 238). Mean TSAT did not change significantly by decade of age for males, while for females mean TSAT was lower for the age group 40 to 49 years (Supplemental Figure 2 in the online supplement).
To better understand non-anemic versus anemic individuals with iron deficiency, clinical characteristics were compared across anemia groups by sex in Supplemental Table 1 in the online supplement. For both sexes, the non-anemic individuals were on average younger, had a higher FEV1 percentage predicted, and a lower prevalence of CHF, CKD, diabetes, and oral anticoagulation use compared to anemic individuals (p≤0.01).
Association Between Transferrin Saturation and All-Cause Hospitalization With Effect Modification by Anemia
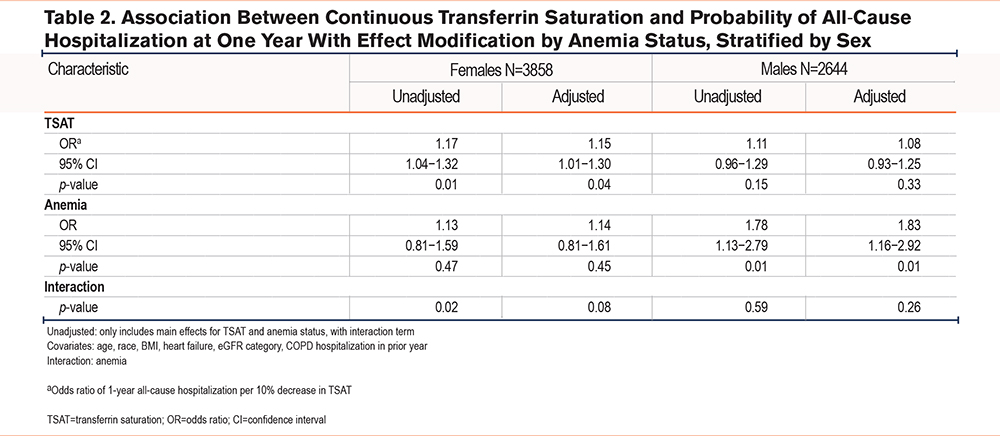
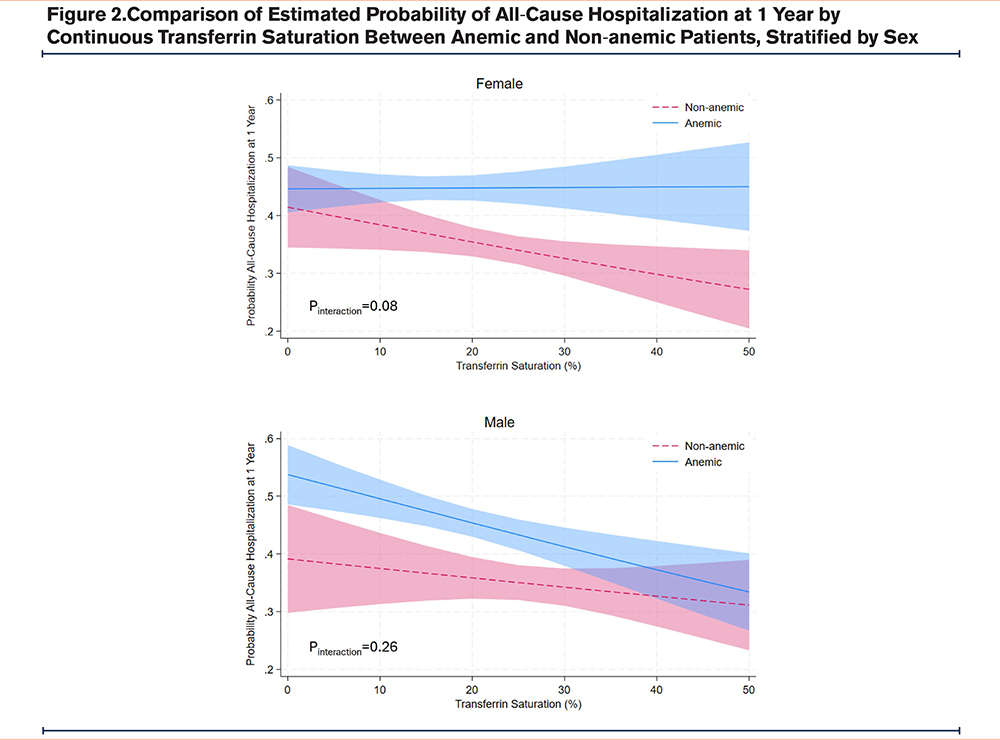
The association between continuous TSAT and all-cause hospitalization is shown in Table 2 A decrease in TSAT by 10% was associated with 14.3% higher odds of all-cause hospitalization in females (95%CI:1.01−1.30, p=0.04), but not in males (odds ratio: 1.08, 95%CI:0.93−1.25, p=0.33). There was effect modification by anemia status such that the association between TSAT and all-cause hospitalization was greater in non-anemic females (p-value interaction=0.08) compared with anemic females (Figure 2). In males, anemia status was a confounder without significant interaction with TSAT (p-value interaction=0.29).
We performed a sensitivity analysis including only individuals with spirometry-confirmed COPD, comprised of 753 females and 672 males (Supplemental Table 2in the online supplement). The trends for the association between TSAT and all-cause hospitalization, and effect modification by anemia status mirrored those observed in the main model above, however, the effect estimates were not statistically significant. Examining the association using iron status categories (deficient versus normal) with all-cause hospitalization, there was no significant association for either sex (Supplemental Table 3 in the online supplement). Anemia was associated with significantly higher odds of all-cause hospitalization for both females (OR 1.69 95%CI 1.4, 2.1, p<0.01) and males (OR 1.36, 95%CI 1.1, 1.7, p<0.01).
Discussion
In this large, diverse cohort of individuals with COPD, we have demonstrated that iron deficiency may be associated with negative outcomes even in the absence of anemia. Importantly, the prevalence of iron deficiency and the impact of iron deficiency on all-cause hospitalizations differed by sex and anemia status; in particular, non-anemic women had the strongest association between TSAT and all-cause hospitalization. These findings suggest certain COPD subpopulations are uniquely affected by iron deficiency which may warrant screening for iron deficiency as part of routine COPD care regardless of anemia status.
Accurately capturing iron deficiency in the presence of chronic diseases such as COPD can be challenging.28 Broadly, iron deficiency is a state of inadequate iron availability relative to the body’s needs. Common causes include blood loss or insufficient dietary intake which leads to absolute iron deficiency (depletion of total body iron stores), while reduced iron absorption and excess sequestration due to inflammation results in functional iron deficiency (insufficient iron delivery, despite adequate iron stores).20 The categories of iron deficiency are not mutually exclusive; multiple etiologies of iron deficiency may coexist, especially in older, multimorbid individuals as in the COPD population. Ferritin, the biomarker conventionally used to define iron deficiency, correlates with total body iron stores in healthy individuals but varies significantly with age, sex, and inflammation.29 As such, ferritin has poor sensitivity to detect both absolute and functional iron deficiency in the COPD population.
The novel, alternative approach applied in this study is the use of TSAT as the primary measure to define iron status in COPD. Prior investigations of iron deficiency in the COPD population have used definitions based on ferritin levels only or a combination of ferritin level and TSAT, with prevalence of iron deficiency ranging from 10%−40%. TSAT, a measure of iron availability that captures both absolute and functional iron deficiency, is less affected by inflammatory states compared to ferritin and has been used to assess iron deficiency in other pulmonary diseases.18,30,31 For example, in a pulmonary hypertension cohort, iron deficiency defined by TSAT was superior to ferritin in identifying patients with altered functional status or exercise capacity as measured by peak oxygen consumption and 6-minute walk distance.32 Furthermore, by handling TSAT as a continuous variable in our main model instead of categorizing iron deficiency by the conventional cutoff of TSAT ≤20%, a larger spectrum of risk could be examined and potential differences in clinically relevant thresholds by sex were not a limitation.
In population-level studies, iron deficiency is generally captured as iron deficiency anemia due to a lack of regular screening in the absence of anemia. However, when iron loss exceeds intake and iron stores become depleted, non-anemic iron deficiency develops before progressing to iron deficiency anemia. While this state is often overlooked, in this study 27% of individuals who were iron deficient were not anemic, and it was the non-anemic subgroup among females in which a clear association between TSAT and all-cause hospitalization was observed.
As iron has functions other than erythropoiesis, iron deficiency may have negative consequences before the onset of anemia.30 This has been shown epidemiologically in CHF and CKD populations where iron deficiency is associated with worse outcomes including higher mortality risk independent of anemia status or type of iron deficiency.26,33 Regarding potential mechanisms, in animal studies iron deficiency without anemia caused a decrease in the activity of iron-containing muscle mitochondrial oxidative enzymes leading to reduced oxidative capacity as well as an imbalance in the energy production systems.34,35 Clinical studies of non-anemic iron deficiency in adults have been limited to studies of young women, but nonetheless have shown that aerobic work capacity was significantly lower in non-anemic iron deficient women after controlling for baseline physical activity level, fat-free mass, and hemoglobin.36
The proposed mechanisms are highly relevant to COPD, where physical activity has been shown to be one of the strongest independent predictors of all-cause mortality.37,38 Also as a proof-of-concept, a small, randomized control trial of intravenous iron for iron deficiency in non-anemic to mildly anemic participants with COPD showed improvement in subjective symptom severity and exercise tolerance.39 While clinical evidence supports iron repletion for systemic iron deficiency as an attractive intervention, the risks of iron overload must also be taken into consideration, particularly in the setting of COPD which is complicated by infectious complications. Mechanistic studies using cell and animal models have demonstrated iron overload in the lungs as a potential driver for COPD pathophysiology through the triggering of dysregulated inflammatory responses, although, there is also conflicting evidence that iron deficiency increases the risk of COPD development in mice.40 In translational studies, bronchoalveolar lavage samples of individuals with COPD have shown elevated iron levels in alveolar macrophages which has been hypothesized to contribute to immune dysfunction.41 Our study results demonstrate the need for further studies to better understand and define iron deficiency at the systemic level in conjunction with organ-level iron status in the lungs to reconcile the evidence from the wide spectrum of research on iron in COPD.
Interestingly, once anemic, TSAT was no longer significantly associated with all-cause hospitalization in either sex. Comparing the anemic and non-anemic individuals who were iron deficient, anemic individuals were older, with worse lung function and greater multimorbidity. Even with similar degrees of iron deficiency by TSAT, anemic individuals may have reached a threshold of severity of chronic illness and multimorbidity such that morbidity and risk of hospitalization are driven by other factors that trump iron deficiency.
Not only was the association of TSAT on all-cause hospitalization modified by anemia status, but a significant association was specifically observed among females. The sex differences in the associations between iron deficiency and outcomes may be in part related to differing COPD phenotypes by sex. There are known differences by sex in patterns of harmful exposures, lung development, lung anatomy, and hormonal effects due to estrogen which together contribute to sex-specific COPD pathophysiology and resulting disease phenotypes. Associated comorbidities also differ by sex, with women tending to have concurrent osteoporosis, malnutrition with lower BMI, and depression/anxiety, whereas men more often have a cardiometabolic constellation of comorbidities such as obesity, diabetes, and atherosclerosis. These 2 patterns of comorbidity clusters in COPD have been well characterized, with a “cachectic” cluster more common in women and a “cardiometabolic” cluster more common in men.42,43 In this study cohort, females had a slightly higher mean BMI compared to males, but the prevalence of underweight BMI was greater among females. The cachectic cluster has been shown to be less inflammatory by serologic markers compared to the cardiometabolic clusters.42 Thus, it is possible that less inflammatory, undernourished iron-deficient women with COPD have a greater component of absolute iron deficiency causing increased morbidity, whereas iron deficiency in men may be primarily functional iron deficiency, a manifestation of inflammation from multiple comorbidities. Another relevant factor specific to females is menopausal status, as iron metabolism is believed to change from the pre- and postmenopausal state; the youngest age group of females in this study cohort had a lower mean TSAT compared to all other age groups. This could not be investigated further in this cohort given the lack of information on menopausal status and the low prevalence of younger women but would be an important area of future study.
The present study has several limitations. First, as a retrospective analysis using EHR data, individuals included in this study had iron measurements obtained for a clinical indication. While screening for iron deficiency is not routine practice in the absence of anemia and could inflate the observed prevalence of iron deficiency, the strength of using a large clinical cohort of individuals with COPD offered real-world insights into iron deficiency in this population. Similarly, the reason iron measurements were obtained for non-anemic individuals is unclear, but more importantly, the prevalence estimate of non-anemic iron deficiency (13.5%), the subgroup of interest that is often missed in routine clinical practice, was likely an underestimate. Second, using EHR data, airway obstruction could be confirmed for only a subset of the cohort. Although the patient characteristics for the subgroup with airway obstruction compared to the whole study cohort were similar, the lack of statistically significant association between TSAT and all-cause hospitalization may be attributed to the smaller sample size and selection bias for unmeasured characteristics introduced by the criteria of having spirometry in the JHHS. Additionally, several factors that may affect iron measures were unable to be obtained from the EHR including red blood cell transfusion history, current smoking status, and burden of previous smoking (pack years). Finally, the study had limited power to investigate the association between TSAT and COPD-specific hospitalizations. The small number of COPD hospitalizations may be due to truly low event rates in this COPD cohort that did not restrict by disease severity or due to encounters not being available through the EHR linked to the COPD PMCOE registry. Of note, the prevalence of individuals with at least one COPD hospitalization in this study cohort was comparable to the prevalence in the overall COPD PMCOE cohort (6.4% versus 6.7%).25 Other than COPD hospitalizations which had been previously validated in this cohort, the outcome was limited to all-cause hospitalization as the EHR did not identify a primary admitting or discharge diagnosis. To better understand the associations of iron deficiency with COPD-specific outcomes, future studies of cohorts with severe COPD or enriched for COPD exacerbation should be pursued.
Interpretation
In conclusion, iron deficiency is an important comorbidity in the COPD population that is nonuniformly associated with increased morbidity. The sensitivity to iron deficiency observed in the non-anemic subpopulation highlights how screening for iron deficiency should not be triggered by the development of anemia but may need to be integrated into COPD management regardless of anemia status. Further, the sex differences in the association of iron deficiency with all-cause hospitalizations add to the evidence of heterogeneous COPD phenotypes in women who may benefit from different interventions. In the future, investigations of the mechanisms by which systemic iron deficiency leads to increased morbidity in COPD in parallel with studies of pulmonary iron dysregulation will aid in clarifying the safest approach to managing this comorbidity in individuals with COPD.
Acknowledgements
Author contributions: YK, NP, and AB, contributed to the conception, design, data analysis, interpretation, and preparation of the manuscript. AF, SR, MM, RW, and NH contributed to data interpretation and revision of the manuscript. All authors have contributed substantially to the manuscript and have reviewed and approved the manuscript before submission for publication. AB had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement: Not applicable
Declaration of Interests
NP declares advisory board participation for GlaxoSmithKline and Verona. The other authors have nothing to declare.