Running Head: Pseudomonas Aeruginosa Colonization in COPD Patients
Funding Support: No financial support was received for this study.
Date of Acceptance: January 16, 2025 │ Online Publication Date: February 5, 2025
Abbreviations: AECOPD=acute exacerbation of COPD; aOR=adjusted odds ratio; BMI=body mass index; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CXR=chest radiographs; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GH=Grantham Hospital; HRCT=high-resolution computed tomography; ICS=inhaled corticosteroid; ILD=interstitial lung disease; IQR=interquartile range; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; OR=odds ratio; QMH=Queen Mary Hospital; SD=standard deviation
Citation: Kwok WC, Tam TCC, Chau CH, Lam FM, Ho JCM. Clinical implications of pseudomonas aeruginosa colonization in chronic obstructive pulmonary disease patients. Chronic Obstr Pulm Dis. 2025; 12(2): 137-145. doi: http://doi.org/10.15326/jcopdf.2024.0582
Background
Pseudomonas aeruginosa is a Gram-negative bacteria reported to have significant prognostic value in chronic respiratory diseases including cystic fibrosis and bronchiectasis. 1-4 Epidemiological studies showed an increasing trend of antimicrobial resistance, including multidrug-resistant isolates in recent years. 5 Pseudomonas aeruginosa is well known to persist and this is attributed to its ability to form antibiotic-resistant biofilms. 6 As such, Pseudomonas aeruginosa can persist in the airways as a bacterial colonizer. Pseudomonas aeruginosa can also lead to airway inflammation and secondary lung damage, resulting in worsening clinical status and progressive disease. 7 In recent years, Pseudomonas aeruginosa has also been recognized to be an important microorganism in patients with chronic obstructive pulmonary disease (COPD). 8,9 Pseudomonas aeruginosa can be isolated in up to 15% of patients with COPD. 10 Pseudomonas aeruginosa colonization is more commonly seen in patients with more severe COPD as defined by the Global initiative for chronic Obstructive Lung Disease (GOLD) stage, 11 with mucoid strains more frequently seen in advanced COPD. 12 Patients with exacerbator phenotype, active smokers, patients who had prior admission to an intensive care unit, patients who received several courses of antibiotics or systemic corticosteroids, and those with concomitant bronchiectasis are also at risk of Pseudomonas aeruginosa isolation. 13 Patients with Pseudomonas aeruginosa isolated during an exacerbation or upon follow-up were found to have more frequent exacerbations, more severe airflow limitation, and higher mortality. 14 Pseudomonas aeruginosa can have different manifestations in patients with COPD, including as a colonizer which has a variable duration of persistence, causing acute exacerbations and it may also cause chronic infection. 15 The dose of inhaled corticosteroid (ICS) was also found to be a potential risk factor for Pseudomonas aeruginosa infection in patients with severe COPD. Despite this, the role of Pseudomonas aeruginosa in COPD remains inconclusive. There are still debates on whether Pseudomonas aeruginosa reflects disease severity in COPD or Pseudomonas aeruginosa causes the accelerated deterioration of COPD. Hence, we conducted the current study to investigate the clinical implications of Pseudomonas aeruginosa colonizationin patients with COPD.
Methods
A prospective study was conducted at Queen Mary Hospital (QMH) and Grantham Hospital (GH), tertiary respiratory referral centers in the Hong Kong West Cluster. Chinese patients aged at or above 40 years, with at least 10 pack years of smoking history, and COPD followed in QMH or GH in the year 2021 were prospectively recruited from the respiratory specialty clinic in QMH/GH at their routine follow-up for COPD, with the recruitment done at their clinical stable state. The diagnosis of COPD was confirmed by spirometry demonstrating postbronchodilator airflow limitation with a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio less than 70%, in line with the latest recommendations in GOLD. 12 Notably, individuals with coexisting asthma, bronchiectasis, and interstitial lung disease were excluded. The diagnosis of bronchiectasis was confirmed if it met the international consensus recommendations on the criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults. 16 Written informed consent was obtained from the patient at the time of recruitment. History taking, physical examination, and blood taking for a complete blood count were performed at the time of recruitment. Sputum microbiology results of the patients for 2021 to 2022 were retrieved. The relevant medical records including demographic data, clinical data /investigations, and medication use were recorded during the recruitment visit. Regular use of ICSs, long-acting beta2-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), theophylline, and roflumilast was defined as continuous use if taken for at least 12 months prior to recruitment. Pseudomonas aeruginosa colonization was defined as the persistence of Pseudomonas aeruginosa in repeated (≥3) sputum specimens or bronchoalveolar lavage taken at a stable state without clinical evidence of infection and tissue damage. 17
Acute exacerbation of COPD (AECOPD) was defined using the latest GOLD recommendations. 12 AECOPD was defined as an acute event characterized by worsening respiratory symptoms beyond normal day-to-day variations, leading to a change in medications. Such symptoms include one or more of the following: (1) increased cough frequency and severity, (2) increased sputum volume and/or changed sputum character, and (3) increased dyspnea that requires medical attention and treatment. 12 Mild AECOPD was defined as AECOPD that was treated with short-acting bronchodilators only. Moderate AECOPD was defined as AECOPD treated with short-acting bronchodilators and oral corticosteroids. Severe AECOPD was defined as an AECOPD requiring hospitalizations or emergency department visits. 12 A clinical stable state was defined as free from AECOPD and any systemic corticosteroid use by more than 90 days. Patients were continued on the standard-of-care treatment from the primary team in charge. The patients prospectively received follow-up after recruitment into the study in the respiratory/COPD specialty clinic in QMH/GH every 16 to 26 weeks for symptoms, COPD control, medication compliance, any AECOPDs, and the date of the first exacerbation, until December 31, 2023.
The primary outcome was the development of any moderate to severe AECOPD. The secondary outcomes included the development of any severe AECOPD, any severe AECOPD that required invasive or noninvasive mechanical ventilation, the annual number of moderate to severe AECOPDs, the annual number of severe AECOPDs, the development of pneumonia, the development of extrapulmonary complications (acute coronary syndrome and ischaemic stroke), and the overall survival.
This study was approved by the University of Hong Kong and the Hospital Authority Hong Kong West Cluster Institutional Review Board (approval reference number: UW 21-172).
Statistical Analysis
The demographic and clinical data were described in actual frequency, mean ± standard deviation (SD,) or median (interquartile range [IQR]). Baseline demographic and clinical data were compared between the 2 groups (with or without Pseudomonas aeruginosa colonization) with independent t-tests or nonparametric tests where appropriate. To identify the association between Pseudomonas aeruginosa colonization and the development of moderate to severe AECOPD, severe AECOPD, and very severe AECOPD that required invasive or noninvasive mechanical ventilation, and the development of pneumonia and extrapulmonary complications (acute coronary syndrome and ischaemic stroke), univariate logistic regression analyses were performed. Multiple logistic regression modeling was used to assess for covariates. Age, gender, body mass index, baseline FEV1, COPD Assessment Test (CAT) scale, number of AECOPDs in the year before participant recruitment, use of relevant medications (ICSs, LAMAs, LABAs), and baseline blood eosinophil count were adjusted as covariates. These covariates were adjusted as they were factors reported to be associated with risks of AECOPD. Multivariable linear regression was performed to assess the association between Pseudomonas aeruginosa colonization and the annual number of moderate to severe AECOPDs/severe AECOPDs. Cox regression analysis was used to assess the survival. Fields with p<0.05 are regarded as statistically significant at the 2-sided test. All statistical analyses were done using the 28th version of the SPSS statistical package (IBM; Armonk, New York).
Results
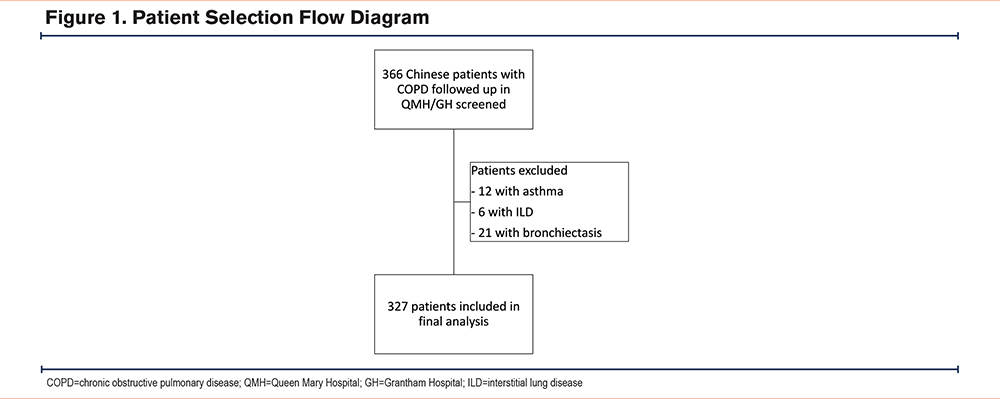
There were 366 patients with COPD followed in QMH/GH who were screened in this study. A total of 12 patients with a history of asthma, 6 with interstitial lung disease, and 21 with bronchiectasis were excluded. The patient selection is illustrated in Figure 1. A total of 327 patients were included in the final analysis.
Baseline Characteristics
The mean age was 74.5±8.9 years, with male predominance (91.1%). The mean FEV1 was 1.40±1.34L (63±23% predicted). The mean CAT score was 9.5±7.7. Among these 327 patients included, 33 (10.1%) had Pseudomonas aeruginosa colonization. Ten (3.1%) of the patients had Moraxella catarrhalis colonization and 42 (42.8%) had Haemophilus influenza colonization. Patients with or without Pseudomonas aeruginosa colonization had similar background characteristics except for a higher number of AECOPDs in the past 1 year before recruitment. The results are summarized in Table 1. The mean follow-up duration was 1.94 ± 0.48 years.
Moderate to Severe Acute Exacerbations of COPD
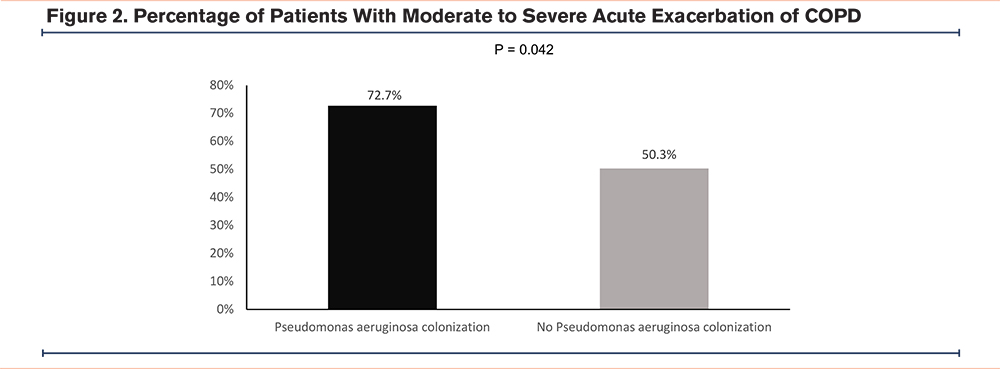
A total of 172 (52.6%) of the patients had moderate to severe AECOPDs in the follow-up period. Twenty-four (72.7%) patients in the Pseudomonas aeruginosa colonization group and 148 (50.3%) in the non-Pseudomonas aeruginosa colonization group had moderate to severe AECOPDs in the follow-up period (Figure 2). The odds ratio (OR) was 2.63 (95% confidence interval [CI] 1.18–5.85, p=0.018) for the Pseudomonas aeruginosa colonization group. The adjusted OR (aOR) was 3.15 (95% CI 1.05–9.48, p=0.042) suggesting increased risks of a severe AECOPD in the Pseudomonas aeruginosa colonization group.
The median annual moderate to severe AECOPD frequency was 0 [0–0.93] in the non-Pseudomonas aeruginosa colonization group and 1.35 [0–3.39] in the Pseudomonas aeruginosa colonization group, with a p-value of 0.005 in multivariable linear regression.
Severe Acute Exacerbations of COPD
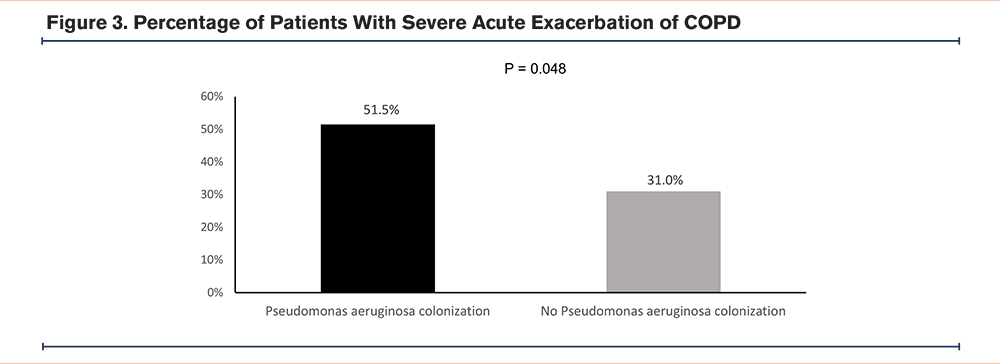
A total of 108 (33.0%) of the patients had severe AECOPDs in the follow-up period. Seventeen (51.5%) patients in the Pseudomonas aeruginosa colonization group and 91 (31.0%) in the non-Pseudomonas aeruginosa colonization group had severe AECOPDs in the follow-up period (Figure 3). The OR was 2.37 (95% CI 1.15 –4.90, p=0.020) for the Pseudomonas aeruginosa colonization group. The aOR was 2.59 (95% CI 1.01–6.64, p=0.048) suggesting increased risks of severe AECOPDs in the Pseudomonas aeruginosa colonization group.
The median annual severe AECOPD frequency was 0 [0–0.46] in the non-Pseudomonas aeruginosa colonization group and 0.43 [0–1.31] in the Pseudomonas aeruginosa colonization group, with a p-value of 0.064 in multivariable linear regression.
Severe Acute Exacerbations of COPD That Required Invasive or Noninvasive Mechanical Ventilation
Twenty-four (7.3%) of the patients had severe AECOPDs that required invasive or noninvasive mechanical ventilation in the follow-up period with 4 (12.1%) in the Pseudomonas aeruginosa colonization group and 20 (6.8%) in the non-Pseudomonas aeruginosa colonization group. The OR was 1.89 (95% CI 0.61–5.91, p=0.27) for the Pseudomonas aeruginosa colonization group.
Pneumonia
Thirty-eight (11.6%) of the patients had pneumonia in the follow-up period with 8 (24.2%) in the Pseudomonas aeruginosa colonization group and 30 (10.2%) in the non-Pseudomonas aeruginosa colonization group. The OR was 2.82 (95% CI 1.17–6.80 p=0.02) for the Pseudomonas aeruginosa colonization group. The aOR was 4.19 (95% CI 1.40–12.54, p=0.011) suggesting increased risks of pneumonia in the Pseudomonas aeruginosa colonization group.
Among the patients who developed pneumonia, 10 (33.3%) in the non-Pseudomonas aeruginosa colonization group had pneumonia due to Pseudomonas aeruginosa and 20 (66.7%) had pneumonia caused by other microorganisms.
Among the patients who developed pneumonia, 3 (37.5%) in the Pseudomonas aeruginosa colonization group had pneumonia due to Pseudomonas aeruginosa and 5 (62.5%) had pneumonia caused by other microorganisms.
Extrapulmonary Complications
Three (0.9%) of the patients developed acute coronary syndrome in the follow-up period with none in the Pseudomonas aeruginosa colonization group and 3 (1.0%) in the non-Pseudomonas aeruginosa colonization group. Three (0.9%) of the patients developed acute coronary syndrome in the follow-up period with none in the Pseudomonas aeruginosa colonization group and 3 (1.0%) in the non-Pseudomonas aeruginosa colonization group.
Mortality
A total of 67 (20.5%) patients died in the follow-up period with 7 (21.2%) in the Pseudomonas aeruginosa colonization group and 60 (20.4%) in the non-Pseudomonas aeruginosa colonization group. There was no statistical difference in the mortality risks in the 2 groups, with a hazard ratio of 1.06 (95% CI = 0.48–2.32, p=0.89) for the Pseudomonas aeruginosa colonization group.
Discussion
In this study, the prognostic role of Pseudomonas aeruginosa colonization was demonstrated. Pseudomonas aeruginosa colonization was shown to be an independent risk factor for future AECOPDs, including moderate to severe AECOPD and severe COPD, as well as pneumonia. The importance of Pseudomonas aeruginosa colonization in COPD should not be underestimated.
Pseudomonas aeruginosa is one of the most important bacterial pathogens in chronic respiratory diseases. In bronchiectasis, Pseudomonas aeruginosa was shown to be associated with increased inflammation, greater impairment of lung function, more exacerbations, increased mortality, and a deterioration of life quality. 1,18,19 Its ability to form biofilms provides Pseudomonas aeruginosa with an enormous advantage in establishing infections. 20 Because of its prognostic value in bronchiectasis, Pseudomonas aeruginosa colonization was included in different scoring systems for the severity assessment of bronchiectasis, including the Bronchiectasis Severity Index,21 the FACED score (FEV1 percentage predicted [F], age [A], presence of chronic colonization by Pseudomonas aeruginosa [C], radiologic extension, [E] and dyspnea [D]) 22 and the E-FACED score (FACED plus exacerbations). 23 Chronic bacterial infection and colonization are also being recognized in COPD. 24 However, one limitation in prior studies was that patients with coexisting bronchiectasis, in which Pseudomonas aeruginosa is commonly colonizing the airway, were also included. 25,26 Hence, it is necessary to understand the burden and clinical implications of bacteria colonization, in particular Pseudomonas aeruginosa colonization, in patients with pure COPD.
In our study, patients with Pseudomonas aeruginosa colonization were shown to have increased risks of moderate to severe AECOPD, severe AECOPD, and pneumonia. This concurs with previous reports on the negative impact of Pseudomonas aeruginosa colonization in COPD and other respiratory diseases. While Pseudomonas aeruginosa eradication has been recommended in bronchiectasis, there has been a lack of evidence on that in COPD and this remains a clinical controversy. 10
The association of Pseudomonas aeruginosa colonization and AECOPD risks can be explained by the nature of Pseudomonas aeruginosa and its effect on the airways. First, Pseudomonas aeruginosa can form a biofilm, which is antibiotic-resistant and offers protection for the bacteria, subsequently allowing colonization in the airway. 6 Pseudomonas aeruginosa has also been shown to contribute to airway inflammation and epithelial damage in bronchiectasis. 4,27 Pseudomonas aeruginosa colonization has been shown to be a risk factor for bronchiectasis exacerbation. 2 These properties of Pseudomonas aeruginosa, as demonstrated in other diseases such as bronchiectasis, could contribute to the observed AECOPD risks in our study. By colonizing the airway in patients with COPD, this leads to chronic airway inflammation, which eventually results in an AECOPD. An AECOPD is one of the most important risk factors for future AECOPDs. 28 Pseudomonas aeruginosa colonization can serve as the trigger for this vicious cycle in COPD. Once colonized in the airway, it is difficult to be eradicated and leads to the inflammatory cascade with AECOPD being the final outcome.
In our study, we did not demonstrate the relationship with ICS use, including the dose and type of ICS associated with risks of Pseudomonas aeruginosa colonization. In prior studies, ICS dose was reported to be associated with increased risks of Pseudomonas aeruginosa colonization. 13 To properly examine this association, a larger sample size may be needed. This possible association is worth further study as ICS use, especially fluticasone, has been demonstrated to be associated with pneumonia risks in COPD. 29 On the other hand,ICS use was shown not to further augment the already increased risk of hospitalization for pneumonia associated with concomitant bronchiectasis in patients with COPD. 30 Nonetheless, safe initiation of ICSs should be executed in patients with COPD especially among those with Pseudomonas aeruginosa colonization given their elevated AECOPD risks.
While we excluded patients with known bronchiectasis in our study, it would be interesting to know if Pseudomonas aeruginosa colonization among COPD patients is linked to future risks of developing bronchiectasis. Further studies are required to determine if COPD patients colonized with Pseudomonas aeruginosa in their airways are more likely to develop bronchiectasis compared with those colonized with other organisms.
Our study has several limitations. First, the study was conducted in 2 tertiary centers and we may have missed those patients with mild COPD who are managed in a primary care setting. Yet, those with milder COPD are also less likely to have AECOPDs and pneumonia, the primary and secondary outcomes of our study. Second, not all patients had high-resolution computed tomography (HRCT) of the thorax to exclude bronchiectasis. The authors reviewed all chest radiographs (CXR) with radiologists reporting the chest radiograph. This may underestimate the number of cases of bronchiectasis that were excluded. As Pseudomonas aeruginosa colonization predominantly occurs in patients with more severe bronchiectasis, and all patients with Pseudomonas aeruginosa colonization had HRCT and/or CXR reviewed to confirm they did not have bronchiectasis, this limitation is a relatively minor one. To further validate the findings of this study, a larger scale study with HRCT completed in all participants should be conducted.
Conclusion
Pseudomonas aeruginosa colonization is an independent risk factor for moderate to severe AECOPD and pneumonia among patients with COPD without coexisting bronchiectasis.
Acknowledgements
Authors' contributions: WCK contributed to the study concept and design, analysis and interpretation of the data, acquisition of the data, and drafting of the manuscript. TCCT and CHC were involved in the critical revision of the manuscript for important intellectual content. JCMH contributed to the study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and was responsible for supervision of the study. All authors provided approval of the final manuscript.
Data sharing: Research data is not available to be shared.
Declaration of Interests
The authors have no conflicts of interest to declare.