Running Head: Hospitalized NTM Pulmonary Disease Patients
Funding support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Date of Acceptance: January 22, 2025 │ Publication Online Date: February 11, 2025
Abbreviations: aIRR=adjusted incidence rate ratio; aOR=adjusted odds ratio; BMI=body mass index; CCI=Charlson Comorbidity Index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; HCUP=Health Care Utilization Project; HMO=health maintenance organization; IRR=incidence rate ratio; ICD-10-CM=International Classification of Diseases, Tenth Revision, Clinical Modification; LOS=length of stay; NTM-PD=nontuberculous mycobacteria pulmonary disease; NIS=National Inpatient Sample; SD=standard deviation
Citation: Baig SH, Sirapu S, Johnson J. Hospitalized nontuberculous mycobacterial pulmonary disease patients and their outcomes in the United States: a retrospective study using national inpatient sample data. Chronic Obstr Pulm Dis. 2025; 12(2): 146-157. doi: http://doi.org/10.15326/jcopdf.2024.0568
Online Supplemental Material: Read Online Supplemental Material (138KB)
Introduction
Nontuberculous mycobacteria (NTM) represent a growing public health challenge, with rising rates of infection increasingly recognized across various populations.1 This trend may be driven by improved diagnostic capabilities, greater exposure to the pathogen, or heightened awareness among health care providers.2 In the United States, from 2008 to 2015, the incidence and prevalence of NTM pulmonary disease (NTM-PD) increased annually by 5.2% and 7.5%, respectively. This growing patient population experiences significant morbidity, elevated health care utilization, and expenditures compared to those without the disease.3 Patients with NTM-PD often endure debilitating symptoms such as chronic cough, shortness of breath, and fatigue, which severely impact their quality of life.4 Recent studies have also indicated a heightened risk of all-cause mortality among these patients relative to age-matched controls.5
Although NTM-PD is typically considered a chronic, progressive condition managed in outpatient settings, these patients are significantly more likely to require hospitalization and have a greater annual hospitalization rate than age- and sex-matched controls.3,6 Given that NTM infections predominantly affect the elderly and immunocompromised,7 it is reasonable to expect that the inpatient burden of NTM-PD will continue to rise as the prevalence of the disease increases.
Despite the substantial evidence highlighting the growing impact of NTM-PD on the health care system, there is a notable gap in research focused on this disease within the hospital setting. Our study seeks to address this gap by characterizing the hospitalized NTM-PD patient population and identifying the factors that influence key hospital outcomes, particularly length of stay and discharge disposition.
Materials and Methods
Study Design and Data Source
We conducted a retrospective cohort study utilizing discharge data from the National Inpatient Sample (NIS), a component of the Healthcare Cost and Utilization Project (HCUP) under the Agency for Healthcare Research and Quality.8 The NIS is the largest, publicly available all-payer inpatient care administrative database in the United States, providing data on over 7 million hospital stays annually from 48 states, covering 97% of the U.S. population. The database includes weighted discharges to represent the entire U.S. hospital discharge population, sampling from all hospitals within HCUP. Each record in the NIS includes up to 40 International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes and 15 procedure codes associated with each hospital stay. The dataset also contains de-identified patient-level information alongside hospital characteristics. The NIS database is de-identified and publicly accessible; as a result, we did not request institutional review board approval or patient consent.
Patients and Variables
Our cohort comprised adult patients aged 18 and older who were hospitalized with NTM-PD. Cases were identified using the ICD-10 code A31.0. The NIS records the principal diagnosis as the first code, followed by secondary diagnoses in numerical order. We included all patients for whom NTM-PD was recorded as the principal diagnosis. Patients transferred between hospitals and those with cystic fibrosis (ICD-10 codes E84.0, E84.1, E84.11, E84.19, E84.8, and E84.9) were excluded from the analysis (See Figure 1 for cohort selection).
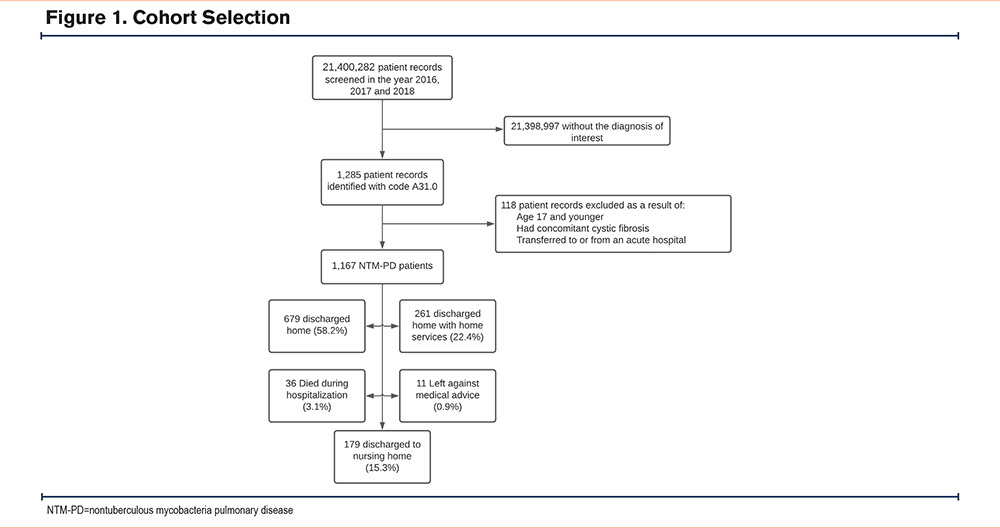
We extracted both patient and hospital characteristics from the NIS to assess various risk factors for 2 primary outcomes: hospital length of stay (LOS) and discharge to a nursing home. Variables of interest included age, gender, race, income status, insurance status, hospital bed size, hospital location, year of admission, number of diagnoses, and number of procedures. The Charlson Comorbidity Index (CCI) was used to risk-stratify the hospitalized patients. The ICD-10-CM codes used to identify chronic medical conditions included in the CCI calculation, as well as other medical conditions of interest, are detailed in Supplementary Table 1 in the online supplement. We conducted exploratory analyses to identify factors associated with an increased LOS among the patient cohort. A significant factor influencing LOS was discharge to a nursing home, prompting us to further analyze the variables associated with this outcome.
Statistical Analysis
Patient characteristics were compared using the Student’s t-test for normally distributed continuous data, the Mann-Whitney U test for nonnormally distributed continuous data, and the Pearson Chi-square test for categorical variables. We performed both univariate and multivariate analyses. Negative binomial regression was utilized for analyzing LOS, while logistic regression was employed to assess the likelihood of discharge to a nursing home. Both models were adjusted for parameters significant in univariate analysis or those with biological plausibility.
The predictors included in the models were age, gender, race, household income, payer status, hospital bed size, hospital location, calendar year, number of diagnoses, number of procedures, CCI, and chronic medical conditions of interest. We calculated adjusted incidence rate ratios (aIRR) for the negative binomial regression and adjusted odds ratios (aOR) for the logistic regression models. A backward conditional stepwise approach was used to develop various iterations of both models, with a p-value of 0.10 as the threshold for inclusion. A 2-sided p-value of <0.05 was considered statistically significant. All analyses were conducted using IBM SPSS Statistics for Windows, version 25 (IBM; Armonk, New York).
Results
Nontuberculous Mycobacteria Pulmonary Disease Patients–Total Cohort
Baseline Characteristics
The study analyzed data from 1167 patients diagnosed with NTM-PD (Table 1). The mean age of the cohort was 66.9 years, with males comprising 44.6% of the population. Most patients were White (66.6%), followed by Black (10.6%), Hispanic (8.6%), and individuals of other racial backgrounds (12.3%). Medicare was the primary payer for 63.7% of these patients, followed by Medicaid (14.0%), private/health-maintenance organization (HMO) insurance (18.2%), and other/self-pay (3.9%). On average, patients had 14.5 diagnoses each. The CCI indicated a significant burden of comorbidities, with 52.6% of patients having a CCI score of 4 or higher. Chronic pulmonary conditions were prevalent, affecting 70.2% of patients, with COPD (39.0%) and bronchiectasis (24.6%) being the most common. Other frequent chronic conditions included hypertension (51.4%), cardiovascular and cerebrovascular disease (24.2%), hypothyroidism (15.3%), and diabetes (14.4%).
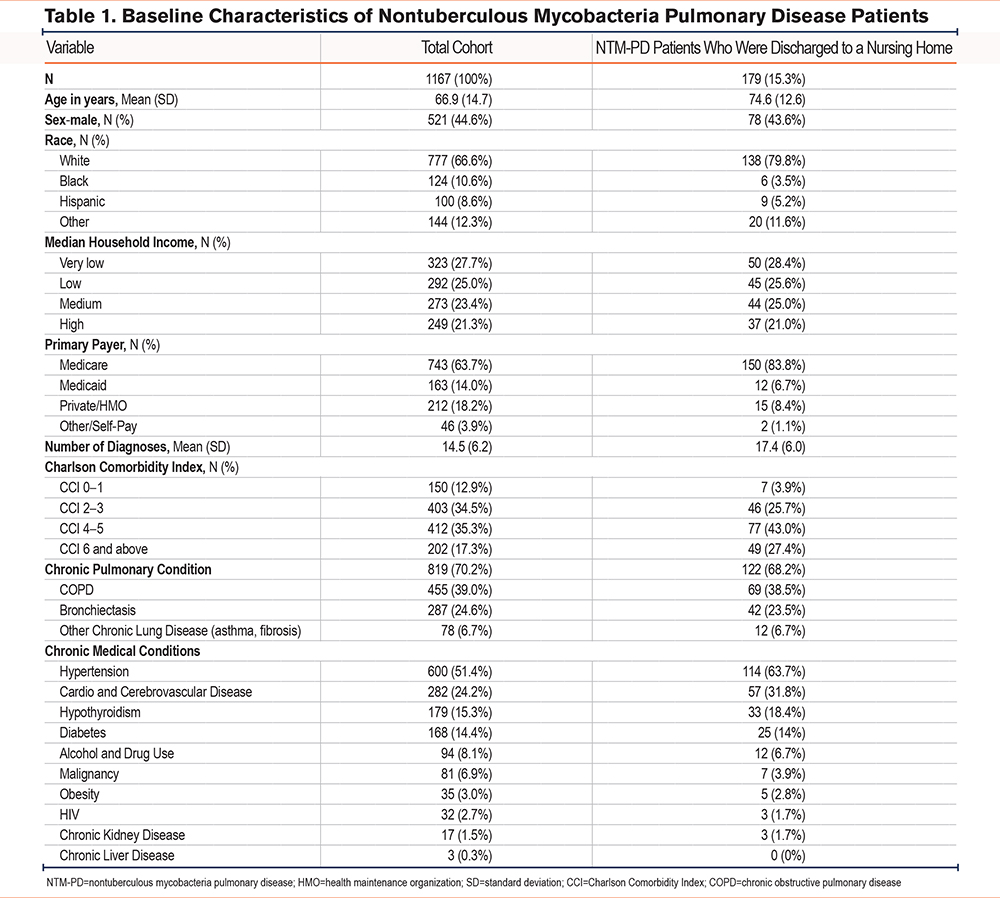
Most NTM-PD patients (75.1%) received treatment in urban teaching hospitals (Table 2). Of the cohort, 57.4% were treated in large hospitals, 27.2% in medium-sized hospitals, and 15.4% in small hospitals. Regionally, the highest proportion of patients was from the South (44.6%), followed by the West (19.7%), Northeast (19.2%), and Midwest (16.5%).
Outcomes
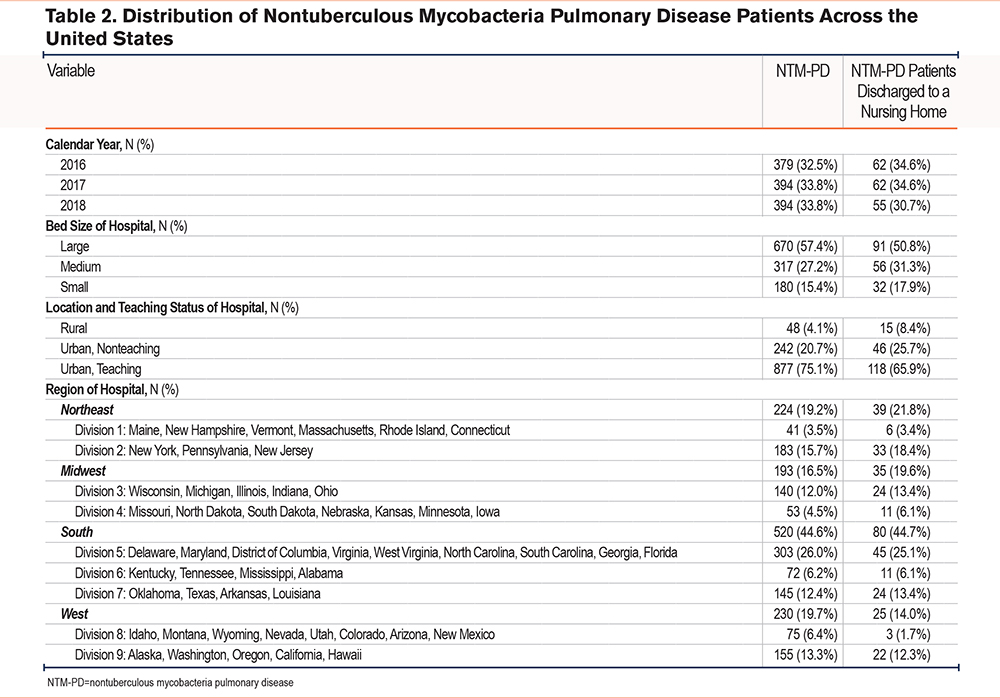
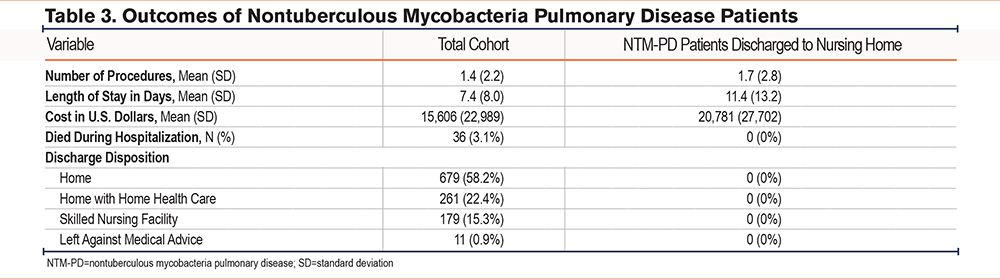
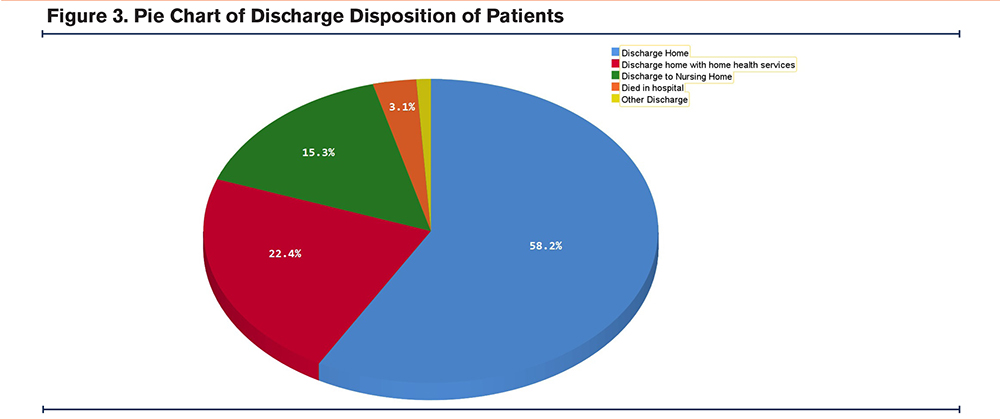
The mean LOS for the total cohort was 7.4 days (Figure 2), with an average of 1.4 procedures performed and mean hospital costs of $15,606 (Table 3). The in-hospital mortality rate was 3.1%. Regarding discharge disposition, 58.2% of patients were discharged home, 22.4% were discharged home with home health care, 15.3% were discharged to skilled nursing facilities, and 0.9% left against medical advice (Figure 3).
Multivariate negative binomial regression identified several predictors that impact LOS among NTM-PD patients (Table 4). Female patients had slightly shorter LOSs than their male counterparts (IRR=0.91, p=0.04). Conversely, patients with private/HMO insurance experienced longer LOSs compared to those with Medicare (IRR=1.18, p=0.007). Patients treated in hospitals located in the Mountain and Pacific region had a 38% (IRR=1.38, p=0.02) and 33% (IRR=1.33, p=0.02) higher LOS, compared to the baseline region of New England. Additionally, we noted a 3% rise in the hospital LOS for each additional diagnosis (IRR=1.03, p<0.0001). Similarly, each additional procedure performed was linked to a 12% increase in the LOS (IRR=1.12, p<0.0001).
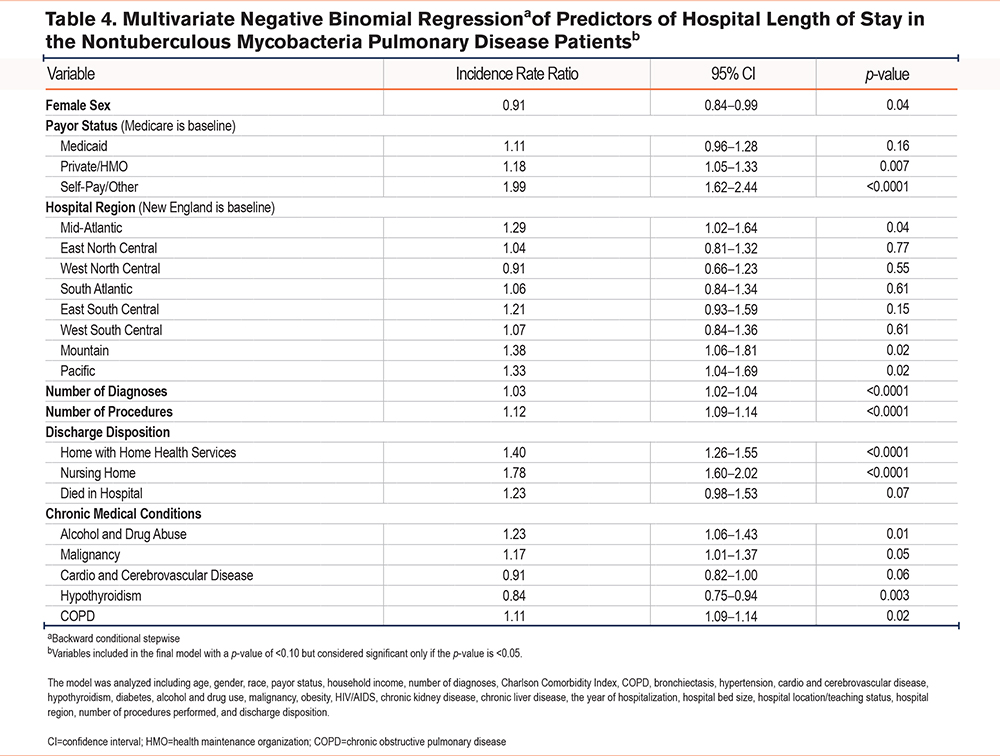
Discharge disposition further influenced the hospital length of stay. Patients discharged to a nursing home had a 78% higher rate of longer stays (IRR=1.78, p<0.0001), and those discharged home with home health services had a 40% higher rate (IRR=1.40, p<0.0001) compared to those discharged directly home. Certain chronic conditions were also predictors of longer hospital stays. Patients with a history of alcohol or drug abuse had a 23% higher rate (IRR=1.23, p=0.01), those with malignancies had a 17% higher rate (IRR=1.17, p=0.05), and those with COPD had an 11% higher rate (IRR=1.11, p=0.02).
Nontuberculous Mycobacteria Pulmonary Disease Patients Discharged to Nursing Homes
Baseline Characteristics
Among the 1167 patients, 179 (15.3%) were discharged to nursing homes. These patients were older, with a mean age of 74.6 years (Table 1). The gender distribution was similar to the total cohort, with males comprising 43.6% of this group. A higher percentage of White patients were discharged to nursing homes (79.8%) compared to the total cohort (66.6%), while the proportions of Black (3.5%), Hispanic (5.2%), and other racial groups (11.6%) were lower. Medicare was the primary payer for a larger proportion of these patients (83.8%), and they had a higher mean number of diagnoses (17.4) compared to the total cohort. Although the prevalence of chronic pulmonary conditions was slightly lower (68.2%), these patients exhibited higher rates of comorbid conditions, such as hypertension (63.7%), cardiovascular and cerebrovascular disease (31.8%), and hypothyroidism (18.4%).
Most patients discharged to nursing homes were treated in urban teaching hospitals (64.9%), with 20.7% treated in nonteaching urban hospitals and 4.1% in rural hospitals (Table 2). Among these patients, 50.8% were treated in large hospitals, 31.3% in medium hospitals, and 17.9% in small hospitals. Regionally, 44.7% of nursing home discharges were from the South, 21.8% from the Northeast, 14.0% from the West, and 19.6% from the Midwest.
Outcomes
Patients discharged to nursing homes had a longer mean hospital stay (11.4 days versus 7.4 days) and incurred a higher average hospital cost ($20,781 versus $15,606) (Table 3) compared to the total cohort.
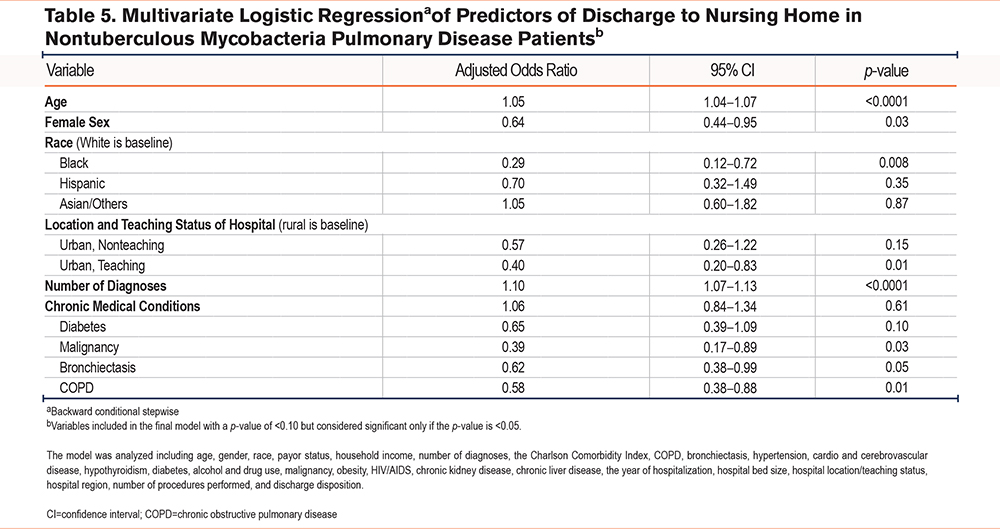
Multivariate logistic regression analysis identified key predictors for discharge to a nursing home (Table 5). Older age was a strong predictor, with each additional year increasing the odds of nursing home discharge by 5% (aOR=1.05, p<0.0001). Females had 36% lower odds of being discharged to a nursing home compared to males (aOR=0.64, p=0.03). Black patients had 71% lower odds of nursing home discharge compared to White patients (aOR=0.29, p=0.008). Hospital characteristics also influenced discharge disposition, with patients treated in urban teaching hospitals having 60% lower odds of being discharged to a nursing home compared to those treated in rural hospitals (aOR=0.40, p=0.01). Each additional diagnosis increased the odds of nursing home discharge by 10% (aOR=1.10, p<0.0001). Among chronic conditions, patients with malignancies had 61% lower odds (aOR=0.39, p=0.03), and those with COPD had 42% lower odds (aOR=0.58, p=0.01) of being discharged to a nursing home.
Discussion
Our study revealed an overall mean hospital LOS of 7.4 days and an average hospitalization cost of $15,606 in NTM-PD patients. Several patient variables were significantly associated with longer LOS, including male gender, private insurance status, a higher comorbidity burden as measured by the CCI, and the need for a greater number of procedures while hospitalized. Among these variables, discharge disposition emerged as one of the strongest predictors of LOS, with discharge to a nursing home associated with a 78% higher LOS. Patients discharged to nursing homes had a mean LOS of 11.4 days and an average hospital cost of $20,781 per stay. Interestingly, while older age was not linked to a longer LOS in the overall cohort, it was a significant predictor of discharge to a nursing home. Additionally, Black and female patients were significantly less likely to be discharged to a nursing home. Among comorbidities, malignancy and COPD were most strongly associated with longer hospital stays. These were protective factors when it came to discharge to nursing homes.
NTM-PD is more prevalent in older adults, females, and White patients1 as reflected in our hospitalized cohort. The literature is unclear about the relationship between household income and NTM-PD, though some studies suggest that factors like increased exposure to air pollution, occupational hazards, poor nutritional status, and a lower body mass index (BMI) may contribute to the incidence of NTM-PD.9,10 However, we found no significant difference in household income within our hospitalized cohort or among those discharged to nursing homes.
The most common nonpulmonary comorbidities in our cohort were hypertension, cardiovascular and cerebrovascular diseases, hypothyroidism, and diabetes. While similar findings have not been extensively reported in the NTM-PD population, given the older age of our cohort, it is not surprising to see high rates of these commonly prevalent conditions.11 The average number of diagnoses per patient was 14.5 in the overall cohort and 17.4 in the subgroup discharged to nursing homes, which is notably higher than the average for a Medicare beneficiary.11
The association between NTM-PD and other airway diseases is well documented.12 Bronchiectasis, a structural lung disease, is a recognized risk factor for NTM-PD,13,14 and the coinfection of COPD patients with NTM is increasingly acknowledged as a cause of uncontrolled symptoms15 and perhaps linked to exacerbations.6 There is a mechanistic overlap between alpha-1-antitrypsin deficiency and NTM infection.16 In our cohort, 39% of hospitalized NTM-PD patients had coexisting COPD and about 25% had bronchiectasis, with similar proportions observed in the subgroup discharged to nursing home. A study by Henkle et al on the natural history of NTM-PD reported that 35% of patients had concomitant radiographic bronchiectasis and 28% had COPD.17
The number of cases identified per year remained stable between 2016 and 2018. The geographic distribution of NTM-PD in our study was consistent with findings from Strollo et al, who reported higher prevalence in the South and coastal states, relative to landlocked Midwestern states.18 The overall mortality rate in our study was 3.1%. This is significantly less than a 2017 study by Spaulding et al that reported an 11% mortality rate during hospitalizations in patients who had clinical mycobacterial isolates.19 This discrepancy may be due to differences in cohort years, with our study focusing on a more contemporary population or the fact that the Spaulding study analyzed microbiologic isolates across the United States and not the clinical disease.
Our overall LOS of 7.4 days is comparable to U.S. and Korean studies reporting 8 and 9 days respectively, but lower compared to some other studies.19,20 For example, a 2013 study by Ringshausen et al21 reported an average LOS of 18.3 days in a German population with pulmonary NTM as their primary diagnosis. A study done by Mirsaeidi et al, covering the years 2001 to 2012, noted an average LOS of 9.2 days.22 The shorter LOS in our cohort may suggest improvements in disease recognition and an emphasis on early discharge. Mirsaeidi and colleagues reported a mean hospital charge of $47,120, with costs rising over time. Our cohort’s mean charge of $71,334 (corresponding to an adjusted cost of $15,606), despite a shorter LOS, highlights the escalating costs of health care, which outpace inflation.
Multivariate negative binomial regression identified several factors significantly influencing LOS in NTM-PD patients. Female sex was associated with a slightly shorter LOS compared to males, possibly due to differences in disease severity or its progression, or differences in social support. Patients with private/HMO insurance and those who were self-pay/other had significantly longer hospital stays compared to those with Medicare, perhaps reflecting differences in care management practices, access to postdischarge resources, or financial constraints delaying discharge. The analysis also revealed that the number of diagnoses and procedures performed during hospitalization was strongly associated with longer LOSs. This is expected, as a higher burden of comorbid conditions and more medical interventions typically extend hospital stays. While 58.2% of patients were discharged home, those discharged to a nursing home or with home health services had LOSs that were 78% and 40% longer, respectively, likely due to the complexities involved in arranging appropriate care.
Chronic medical conditions such as alcohol and drug abuse, malignancy, and COPD were associated with 23%, 17%, and 11% longer hospital stays, respectively. The link between alcohol use and lung health is increasingly recognized, with alcohol use disorder associated with higher risks of pneumonia,23 tuberculosis,24 and acute respiratory distress syndrome.25 The mechanisms are thought to be mediated through alcohol-related immunosuppression or modulation.26 Malignancy’s impact on the immune system likely contributed to a longer LOS, as it is a known risk factor for NTM-PD.27 The higher LOS in NTM-PD patients with COPD is likely related to underlying lung disease exacerbation. COPD is a recognized risk factor for NTM-PD27 due to structural lung disruption, immune system impairment,15 or corticosteroid use (inhaled or oral)28 to manage underlying disease.
Conversely, hypothyroidism was associated with a shorter LOS, a finding not replicated in other studies and difficult to explain without further data. It is possible that hypothyroidism may reflect a favorable profile of social determinants of health.
In the subgroup analysis of patients discharged to nursing homes, increasing age, male gender, and White race were strong predictors. Older age is an established factor29 in nursing home placement due to increased frailty, comorbidities, and decreased ability to live independently. While previous studies show that females are more likely to be discharged to a nursing home30 and make up most nursing home residents,31 our NTM-PD population indicates that males are more likely to require nursing home care. This disparity may be linked to inherent gender-based differences in the presentation of this disease. Park et al showed that the mean age at the time of diagnosis in women was 60 and the mean age in men was 67 in a South Korean population.32 Perhaps males are diagnosed at a more advanced disease stage resulting in high morbidity and the need for nursing home care. Non-White individuals are less likely to be placed in nursing homes, potentially due to socioeconomic factors, cultural preferences for family-based care, and distrust of institutional care settings.
Hospital characteristics also influenced discharge outcomes. Patients treated in urban teaching hospitals had 60% lower odds of being discharged to a nursing home compared to those treated in rural hospitals, possibly due to greater access to specialized care and better discharge planning resources. The number of diagnoses, as a proxy for overall illness severity, was also a significant factor.
Interestingly, the presence of malignancy was associated with a decreased likelihood of discharge to a nursing home. This counterintuitive finding might reflect the intensive outpatient care required for malignancy, leading to better-prepared discharge plans and alternatives to nursing home care. COPD and bronchiectasis were also linked to lower odds of discharge to a nursing home. Patients with these conditions often return to their baseline symptom burden after hospitalization and are discharged home, which aligns with real-world experience. This finding highlights the importance of effective management of airway diseases to prevent institutionalization.
Population-based data on hospitalizations for NTM-PD is currently limited, making our study a valuable contribution to understanding the clinical characteristics and outcomes of this patient population. As NTM-PD becomes more common, the insights provided by this study can help health care providers optimize patient care and allocate resources more effectively. Although mortality among NTM-PD patients is generally low, hospitalizations for this condition represent a significant economic burden, primarily due to extended lengths of stay and complicated discharge dispositions. This underscores the importance of strategies aimed at preventing hospitalization through frequent follow-up and proactive management of comorbidities, particularly in patients with other pulmonary conditions such as COPD and bronchiectasis.
The significant role of discharge disposition in influencing LOS highlights the necessity of early and coordinated discharge planning, especially for patients likely to be discharged to nursing homes (older males with private insurance status, and a high comorbidity burden). Additionally, the rising costs of hospitalization despite shorter LOSs suggest that health care systems must implement cost-effective strategies that maintain quality care while controlling expenses.
A recent study by Aliberti et al33 highlighted that one-third of patients undergoing treatment for NTM-PD experienced an unsuccessful outcome, including treatment cessation and disease recurrence. This finding underscores the critical importance of effective outpatient management, which, if improved, could lead to a reduction in hospitalization rates for NTM-PD. Overall, a multidisciplinary approach involving early diagnosis, tailored treatment, proactive discharge planning, and ongoing outpatient care is essential to enhance patient outcomes and reduce the burden of NTM-PD on health care systems.
There are several limitations in our study. First, we rely on ICD-10 coding to create our patient sample, which is limited in its ability to classify the dynamic problems faced during a hospitalization. However, a study reported by Mejia-Chew et al suggested a good positive predictive value of code-based identification.34 Of note, their study was done using ICD-9 codes. Code-based administrative studies are prone to provider error and are not meant to replace validated diagnostic criteria. Our study included NTM-PD patients with the principal diagnostic code during hospitalization. Our study likely underestimates the problem, but we still think it provides us with key insights into this patient population. The retrospective study design limits adjustment for residual confounders. Due to limitations of the dataset, we were unable to assess a patient’s BMI, smoking status, baseline therapy, etc., as potential factors that will impact hospital outcomes. Similarly, understanding pre-admission living status would have informed the noted association between discharge to a nursing home and longer LOS. The de-identified nature of the dataset limits our ability to track patient readmissions. Although NTM-PD is not typically associated with frequent readmissions, we estimate that approximately 5% of the entries may represent repeat hospitalizations for the same patient. This minor overlap is unlikely to significantly affect the overall results or the primary focus of our study, which is to analyze and discuss NTM-PD hospital outcomes.
Our study highlights patient variables that contribute to LOS in hospitalizations for NTM-PD. More attention needs to be paid to the clinical characteristics of patients in the outpatient setting that predispose them to hospital admission so that care can be more specifically targeted.
Conclusion
Our study provides valuable and novel insights into the factors influencing hospital LOS and discharge outcomes in patients with NTM-PD, a condition that is becoming increasingly prevalent but remains under-researched in terms of hospitalization data. By identifying key variables such as male gender, private insurance status, and comorbidity burden that contribute to extended LOSs, our findings underscore the importance of personalized and proactive management strategies for NTM-PD patients. The study also highlights the critical role of discharge planning, particularly for older males and patients with a complex medical profile, who are more likely to require nursing home care. Overall, this research not only sheds light on the significant economic and clinical burden of NTM-PD hospitalizations but also emphasizes the need for targeted interventions in the outpatient setting to prevent hospital admissions and improve patient outcomes. These findings lay the groundwork for future studies and interventions aimed at optimizing care for this vulnerable population.
Acknowledgements
Author contributions: All authors contributed to the study. JJ and SS were directly involved with literature review, writing, visualization, and project administration. SB was involved with conceptualization, methodology, software, formal analysis, writing, validation, visualization, and supervision. All authors approved the final version of the manuscript submitted for publication.
Declaration of Interest
SB has received consulting fees from Ubie Pte, Regeneron, and Verona Pharmaceuticals. The remaining authors have no conflicts of interest to disclose.