Running Head: Impact of Inpatient COPD Care Pathway on Outcomes
Funding Support: None
Date of Acceptance: May 30, 2025 | Published Online: June 9, 2025
Abbreviations: ABG=arterial blood gas; ARF=acute respiratory failure; BA=business associate; BH=Bridgeport Hospital; BiPAP=bilevel positive airway pressure; BMI=body mass index; BMP=basic metabolic panel; BNP=B-type natriuretic peptide; BPAP=bilevel positive airway pressure; CBC=complete blood count; CHF=congestive heart failure; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CPAP=continuous positive airway pressure; CPs=care pathways; CT=computed tomography; CXR=chest x-ray; DFA=detrended fluctuation analysis; DNR/I=do not resuscitate/intubate; DME=durable medical equipment; DVT=deep vein thrombosis; ED=emergency department; EHR=electronic health record; EKG=electrocardiogram; FEV1=forced expiratory volume in 1 second; FiO2=fraction of inspired oxygen; FVC=forced vital capacity; GH=growth hormone; GOC=goals of care; GOLD=Global initiative for chronic Obstructive Lung Disease; H&P=history and physical; HS Troponin=high sensitivity troponin; HTN=hypertension; ICD-10-CM=International Classification of Diseases, Tenth Revision, Clinical Modification; ICU=intensive care unit; IQR=interquartile range; LM=Lawrence and Memorial Hospital; LMH=Lawrence and Memorial Hospital; LMW=Lawrence and Memorial Hospital and Westerly Hospital; LOS=length of stay; MICU=medical intensive care unit; mMRC=modified Medical Research Council; NIPPV=noninvasive positive pressure ventilation; NPO=nothing by mouth; OR=odds ratio; OT=occupational therapy; PaCO2=partial pressure of arterial carbon dioxide; PCO2=partial pressure of carbon dioxide; PCP=primary care physician; PCU=progressive care unit; PE=pulmonary embolism; PFT=pulmonary function test; PR=pulmonary rehabilitation; PRN=as needed; PT=physical therapy; RR=respiratory rate; SDU=step down unit; SLP=speech-language pathology; SRC=St. Raphael Campus; VTE PPx=venous thromboembolism prophylaxis; WH=Westerly Hospital; YNH=Yale-New Haven; YNHH=Yale-New Haven Health; YNHHS=Yale-New Haven Health System; YSC=York Street Campus
Citation: Kim N, Teng W, Akande O, Rhodes D, Rochester CL. Impact of an inpatient COPD care pathway on hospital care process and outcome metrics. Chronic Obstr Pulm Dis. 2025; 12(4): 304-316. doi: http://doi.org/10.15326/jcopdf.2024.0585
Online Supplemental Material: Read Online Supplemental Material (192KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow obstruction that leads to increased work to breathe, exercise intolerance, and impaired quality of life.1 Acute exacerbations are a leading cause of hospitalizations.1 Inpatient care during COPD admissions, however, remains variable.2 Moreover, COPD is often misdiagnosed3-5 as comorbidities presenting with similar symptoms are common. Gaps exist between published guidelines for management of acute exacerbations and practice patterns, with low rates of appropriate medication prescriptions and referrals to tobacco cessation or pulmonary rehabilitation as examples.6-9 Suboptimal adherence to evidence-based practice in hospital care may contribute to readmissions and high health care costs.10,11 Multiple strategies have been employed to promote evidence-based care and improve hospital outcomes in COPD, including the use of care navigators, care bundles, and discharge order sets with variable benefits for reducing readmissions or quality of life.12-19 Barriers to care bundle implementation include resource and time constraints, suboptimal staff engagement, and unclear ownership of tasks.12,20
Care pathways (CPs) represent another strategy to enhance adherence to best practice without requiring intense education for providers, time, or extra personnel for implementation.21 By optimizing adherence to evidence-based practice, such pathways also have the potential to reduce hospital readmissions and health care costs for COPD.15,22-25 CPs at their core represent multidisciplinary plans that provide real-time decision support by integrating guidelines and best evidence at the point of care. By sharing a continuous cognitive architecture that can be followed by any care provider at any time, CPs can standardize care and support tailored decisions within a large health care system. Additionally, when integrated within the electronic health record (EHR), CPs allow physicians to perform supported tasks, place orders, and document notes in one sitting without interrupting daily workflows.
The effectiveness of CPs depends on many factors, including establishing the correct diagnosis, provider engagement, and meaningful use of pathways. Most previous studies of CPs in patients hospitalized for COPD have not been structured for actionable use at the point of care.26-28 The aim of this study was to evaluate the impact of a 2-part inpatient COPD CP embedded in the EHR in real-time across a large health care system. We hypothesized that the use of the CP would streamline resource use and improve both process and clinical outcomes related to the hospital admission.
Study Design and Methods
This study was approved by the Institutional Review Board of Yale University which granted a waiver of informed consent (Protocol #2000032391).
Design, Setting, Patients
The Yale-New Haven Health System (YNHHS) is a large health care system with 5 delivery networks comprised of 7 hospitals in Connecticut and Rhode Island, ranging from small community hospitals to large tertiary care academic centers. We conducted a retrospective cohort study of all inpatient adults aged 18 or older who were hospitalized at YNHHS for a coded primary discharge diagnosis of COPD exacerbation or primary diagnosis of acute respiratory failure with a secondary diagnosis of COPD from June 1, 2021, to May 30, 2022. This was the 12-month time period after the implementation of the COPD pathway. We defined the cohort using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes in accordance with methods used by the Centers for Medicare & Medicaid Services (full list of diagnosis codes shown in Supplemental Table 1 in the online supplement). Unique individuals could have contributed more than one index hospitalization.
Intervention
A 2-component COPD CP for inpatient care was developed by local clinical consensus from multidisciplinary stakeholders across the YNHHS including, hospital medicine, pulmonary and critical care medicine, palliative care, respiratory therapy, nursing, pharmacy, physical therapy, care management, and social work. Pathway content was based on current evidence-based COPD care as recommended in the Global initiative for chronic Obstructive Lung Disease (GOLD) report.1 The pathways are provider-level interventions which, if effective, will generate patient-level outcomes. The COPD pathway was embedded into the clinical EHR on May 11, 2021, at all 7 hospitals across the YNHHS and developed using proprietary software from AgileMD, Inc., (San Francisco, California). The pathway was socialized with YNHHS providers through multiple mechanisms including, e-mail communication, newsletters, dedicated educational conferences, ad-hoc meetings, rounding to influence, and word-of-mouth. YNHHS providers had also been familiarized previously with the existence of a CP for the management of COVID-19. We collected data in the 12 months after the pathway went live to allow for a run-in phase concurrent with the promotion campaign.
The pathway provided clinical decision support in real time and allowed providers to document and place orders directly from the pathway. Since hospitalized patients commonly receive care from different providers on the day of admission versus subsequent hospitalization days, the pathway was divided into 2 parts for ease of use. The first component focused on the admission of patients with suspected acute COPD exacerbation (Figure 1). Guidance to confirm the COPD diagnosis with inpatient spirometry or prior computed tomography (CT) imaging was provided since there is often a broad differential for the associated symptoms, and many hospitalized patients have never had COPD confirmed with spirometry.29,30 Criteria for specialty and support referrals were established based on evidence and consensus, including consultation with clinical experts, i.e., palliative care, pulmonary medicine, hospital medicine, respiratory therapy, social work, care management, and ancillary services (e.g., physical therapy, nutrition, etc.) Suggested indications for palliative care included: (1) ≥ 2 admissions for COPD in the last year, (2) a history of refractory or repeated respiratory failure despite home bilevel positive airway pressure or home mechanical ventilation, (3) refractory symptoms and/or progressively declining functional status, or (4) concurrent advanced comorbidities. Suggested indications for pulmonary consult included: (1) ≥ 2 admissions for COPD in the last year, (2) a history of respiratory failure with prior intubation or noninvasive ventilation, (3) unclear diagnosis, (4) not improving in 2 days, (5) questions about medications and/or therapeutic adjustments, (6) a COPD diagnosis and <40 years old, or (7) palliative care consult indicated.
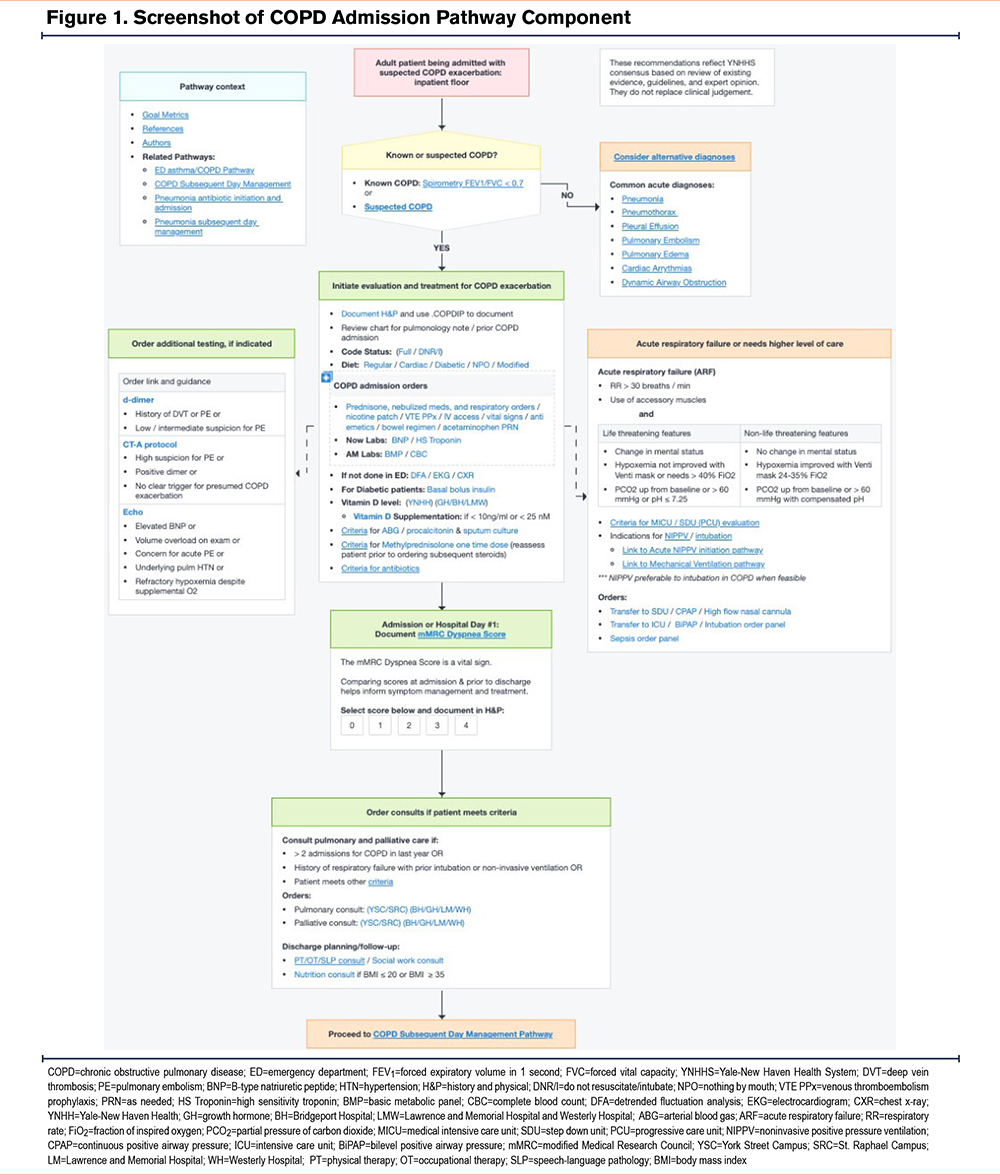
The subsequent day CP component focused on patient education, functional disability (including need for rehabilitation services), need for specialty care referrals and/or social work services, and assessment of need for supplemental oxygen and/or presence of ventilatory insufficiency requiring noninvasive ventilation (Figure 2). This component again reminded providers to confirm the COPD diagnosis, anticipate discharge needs, schedule follow-up appointments, and refer to pulmonary rehabilitation (PR) and tobacco cessation programs or other specialty services. In so doing, the pathway promoted patient-centric care and provided resource stewardship since patients without confirmed COPD should not receive nonbeneficial treatments, e.g., steroids or inappropriate referrals.
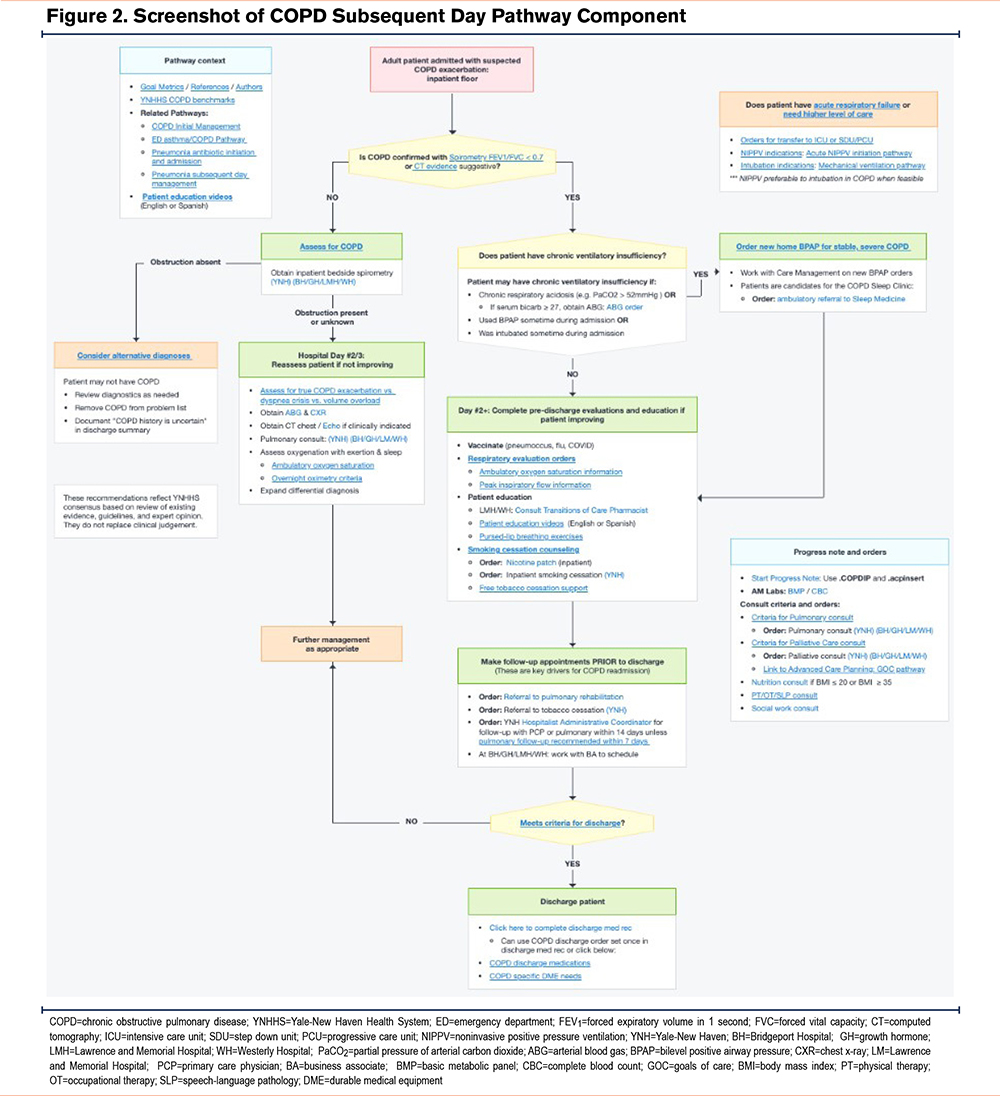
While pathways had to be activated voluntarily by providers, the COPD pathway was suggested within the patient chart when certain criteria were met, such as receipt of both steroids and nebulized anticholinergic medications. All providers, including medical trainees, hospitalists, respiratory and other specialists, and allied health professionals had access to the pathway. All actions available on the pathway were also available through standard order entry off the pathway, e.g., inpatient consult to pulmonary medicine.
Outcomes
Utilization and Clinical Outcomes
Because providers had to enter the pathway intentionally, pathway utilization was measured. Utilization was defined as any opening of either COPD pathway component during hospitalization. It was possible for clinicians to use either the admission or the subsequent day pathway in isolation or together. Opening the pathway required providers to click either a suggestion bar or the pathway tab to locate the COPD pathway. There was no incentive to opening a pathway unless there was intent to use the pathway for any number of activities including: medical decision support, placing orders (for medications, diagnostics, consults or ambulatory referrals), documenting notes, launching patient education videos, accessing scripts regarding tobacco cessation, operational guidance (for how to measure an ambulatory saturation), and general knowledge transfer (how to verify a COPD diagnosis). To define meaningful use, the number of Vitamin D orders was also compared between admissions where the pathway was and was not opened. Vitamin D is included in best practices on the pathway, but not routinely measured for COPD admissions, as it does not directly treat an acute COPD exacerbation. Therefore, Vitamin D orders were a surrogate to demonstrate the pathway was used and not merely opened in the EHR.
Because pathways were designed to improve the process measures that are felt to be key drivers of clinical outcomes, the primary outcome of the study was referral to PR, an intervention associated with decreased mortality and reduced hospital readmission risk in COPD.24,25,31 The prespecified secondary clinical outcome was discharge to home with or without homecare services, a clinical outcome of importance to patients and a leading strategy to control postacute costs.32 Exploratory analyses focused on the association of the COPD pathway use with tobacco cessation referrals, length-of-stay (LOS), and 30-day all-cause readmission.
Standardized Cost
Standardized costs were calculated using a proprietary method from Strata Decision Technology (Chicago, Illinois). Strata provides the financial analytics, planning, and performance platform used by YNHHS. Data from Strata include information standard to hospital discharge files, as well as date-stamped logs of all billed items such as medications, laboratory tests, diagnostics, and therapeutic services. Strata contains costs at the item level and total hospitalization costs. These costs are calculated using accounting methods to allocate hospital incurred expenses to billed items. As such, Strata data are a reasonable proxy for utilization.
Statistical Analysis
Sociodemographic characteristics analyzed included age, sex, race/ethnicity, insurance status, and current tobacco use.31 Asian/Pacific Islander and American Indian/Alaska Native were grouped in the “Other” category because of small numbers. We analyzed race, ethnicity, and insurance status features to assess if there were any disparities in pathway use related to these factors. The Charlson comorbidity index was calculated and assigned based on ICD-10-CM secondary diagnosis codes. Discharge to home included those discharged with and without homecare services in the multivariable analysis. Descriptive statistics are presented as median (interquartile range) for continuous variables and proportions for categorical variables.
Multivariable logistic regression models were constructed to determine the contribution of COPD pathway use on the primary outcome of PR referral and the secondary outcome of discharge to home with or without homecare services. Statistical analyses were conducted in IBM SPSS statistics, Version 26.0 (IBM Corp.; Armonk, New York) and p≤0.05 was considered statistically significant for both outcomes of PR referral and discharge to home.
Results
Pathway Use and Patient Characteristics
A total of 766 unique patients contributed 971 COPD hospitalizations. Any COPD pathway component was opened in 142 (14.6%) of hospitalizations. For all COPD hospitalizations, the median patient age was 65 years, 590 (60.7%) were female, 627 (64.6%) were non-Hispanic White, 221 (22.7%) were non-Hispanic Black, 92 (9.4%) were Hispanic, and 31 (3.2%) were classified as Other. Primary insurance was identified as 377 (38.8%) with private insurance, 266 (27.4%) with Medicare, 312 (32.1%) with Medicaid, and 16 (1.6%) with Other. There were no statistically significant differences regarding demographics or primary insurance between those hospitalizations with versus without pathway use (Table 1). Patients for whom the COPD pathway was accessed did not differ in current, past, or never-smoking status (p = 0.12); Charlson comorbidity scores (mean, 3.8 versus 3.9, p=0.65); or ≥ one admission in the prior year (p=0.36) compared with those who never had the COPD pathway accessed. Although the pathway used group was less likely to have a history of congestive heart failure (CHF), no other differences were noted in the prevalence of individual comorbidities between groups.
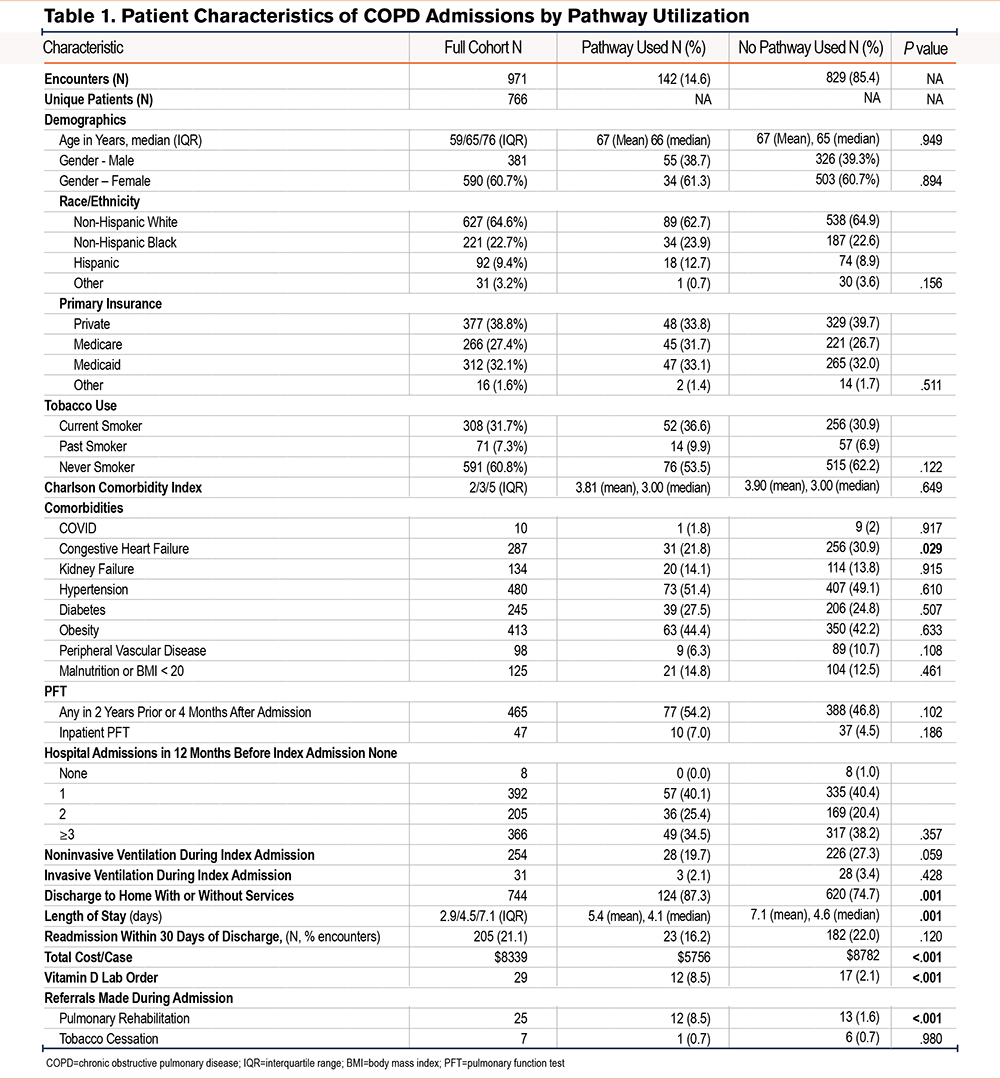
Association Between Pathway Use and Outcomes
In bivariate analyses, referrals to PR were more frequent (8.5% versus 1.6%, p<0.001) among hospitalizations with pathway use, but referrals to tobacco cessation remained low in both groups (0.0% versus 0.6%, p=0.54). The proportion of patients discharged to home with or without homecare services was greater (87.3% versus 74.7%, p=0.001) with versus without pathway use. The LOS was lower (mean, 5.4 days versus 7.1 days, p=0.001) for those hospitalizations with versus without pathway use. Although 30-day readmissions were also lower among the pathway group, this difference was not statistically significant (16.2% versus 22.0%, p=0.12). Vitamin D orders were also higher in the pathway group (8.5% versus 2.1%, p<0.001).
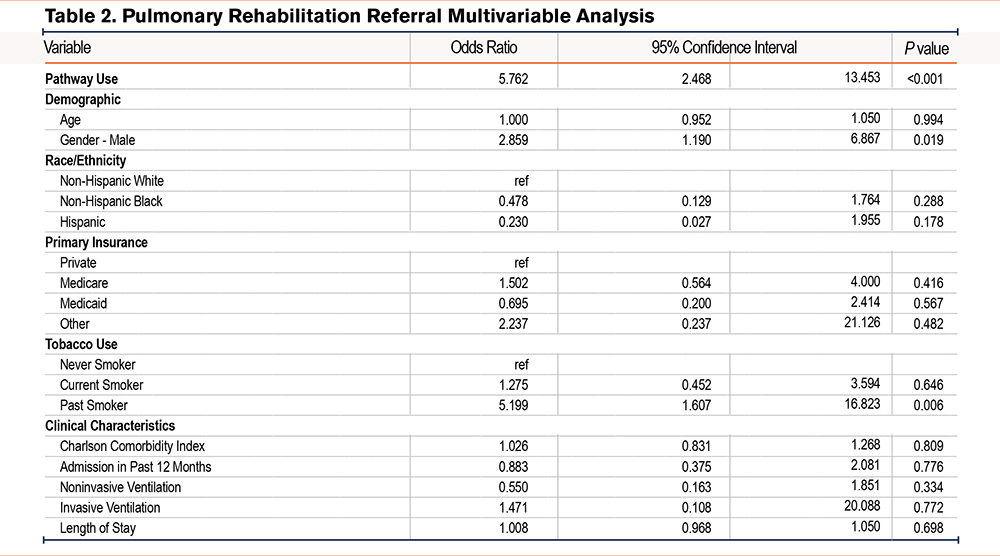
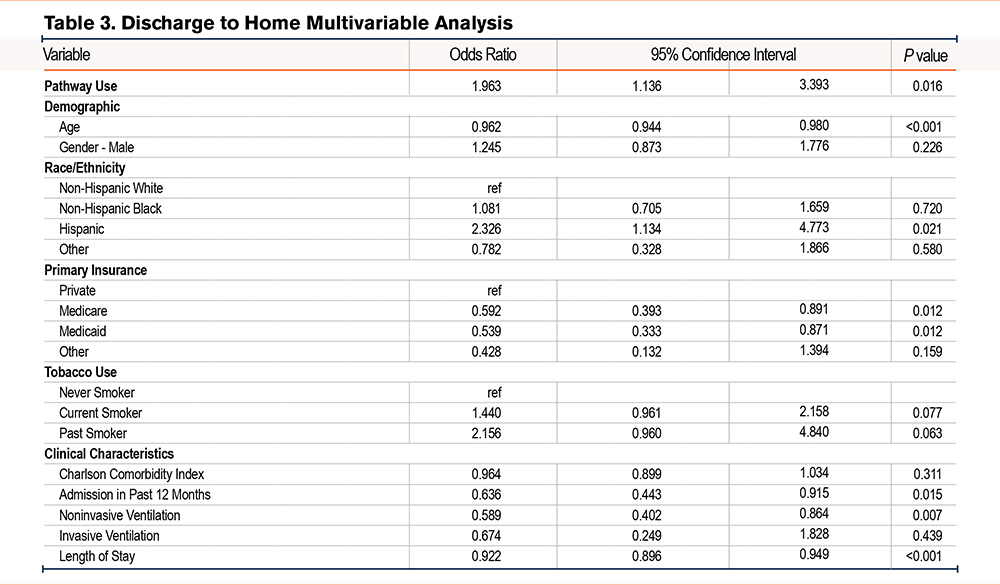
In multivariable analysis, admissions where the pathway was used had greater odds of receiving a referral to PR when compared to admissions without pathway use (odds ratio [OR] 5.76, 95% confidence interval [CI] 2.47–13.4) (Table 2). Other factors associated with increased odds of receiving a PR referral included male gender (OR 2.86, 95% CI 1.19–6.87) and history of smoking (OR 5.12, 95% CI 1.61–16.8). Pathway use was also associated with greater odds of discharge to home with or without services (OR 1.96, 95% CI 1.14–3.39) (Table 3). Other characteristics associated with greater odds of home discharge included Hispanic ethnicity (OR 2.33, 95% CI 1.13–4.77). Variables associated with lower odds of home discharge included age (OR 0.96, 95% CI 0.94–0.98), Medicare (OR 0.59, 95% CI 0.39–0.89), Medicaid (OR 0.54, 95% CI 0.33–0.87), admission in past 12 months (OR 0.64, 95% CI 0.44–0.92), use of noninvasive ventilation during admission (OR 0.59, 95% CI 0.40–0.86), and LOS (OR 0.92, 95% CI 0.90–0.95). Additional multivariable analyses explored the association of the pathways with LOS and 30-day readmissions as outcomes but did not find a statistically significant association (data not shown).
Association Between Pathway Use and Standardized Cost
To better understand resource utilization between COPD admissions where the pathway was and was not used, we examined the percentage of the total cost of hospitalization by different service areas (e.g., labs, diagnostic imaging, therapeutic services, etc.). Bivariate analysis showed that total standardized costs were lower in the pathway group ($5756 versus $8781, p<0.001), with statistically significant differences in cost of pharmacy, labs, diagnostic imaging, blood products, and therapeutic services, which include items such as physical and occupational therapy and endoscopic swallow evaluation. A list of the top items in each category is provided in Supplementary Table 2 in the online supplement.
Discussion
This study highlights the feasibility of implementing a COPD-focused inpatient CP in the EHR across a large health care system. Despite a relatively low utilization rate, pathway use during hospitalization for COPD exacerbation was associated with increased patient referrals to PR, increased discharges to home, with a nonstatistical trend toward lower total standardized hospitalization-related costs, lower LOS, and 30-day readmissions. The pathway was built into the EHR over several weeks using proprietary software from AgileMD, Inc. (San Francisco, California) and evidence-based content was developed by the YNHHS multidisciplinary COPD Clinical Consensus Group. The CP was a minimal cost to the health care system, and no dedicated personnel, such as nurse navigators, were required for its implementation or maintenance.
Several patient-, provider-, and health care system-related factors are associated with hospitalization risk for people with COPD.33-35 Some of these are potentially modifiable, including confirmation of an accurate diagnosis, optimization of pharmacotherapy, supplemental oxygen and/or noninvasive ventilation, tobacco cessation, and improvement in physical function. Correctly identifying COPD is an essential first step in guiding acute management at admission, informing subsequent inpatient care, and coordinating discharge plans. Confirming COPD also avoids the use of inappropriate treatments that may pose risks to patients, incur unnecessary costs, and prolong LOS. Since many conditions mimic COPD and COPD exacerbation, our pathway focuses on verifying the underlying COPD diagnosis, as well as discriminating the causes of acute changes in patients’ symptoms resulting in hospitalization. An order for inpatient spirometry (done in the pulmonary function lab by certified pulmonary function test [PFT] technicians) was available for individuals without any prior confirmation of the COPD diagnosis. Because the diagnosis can be established with historical PFTs and CT scans prior to the index admission, which would obviate the need for inpatient PFTs, we were unable to measure accurately those patients who had COPD confirmed prior to versus at the time of pathway use.
Interestingly, prior qualitative pathway research found that providers worried about “tunnel vision”36 where clinicians anchor to a specific diagnosis without considering alternatives. We found evidence to support the contrary. The COPD pathway was opened on patients who were not discharged with a diagnosis of COPD (data not shown), so were not included in our study cohort. This suggests, however, that some clinicians may have questioned the presumptive diagnosis of COPD and changed course after following the pathway, which explicitly lists a brief differential for shortness of breath that can be confused with COPD, e.g., CHF and pulmonary embolism. Because of known diagnostic misclassification of COPD,3-5 the first step in our pathway can reduce the heuristic fallacy and “tunnel vision” of an initial diagnosis that is often continued when patients transition from the ED to the inpatient medical ward, between day and night staff, and at discharge.
The existing literature around CPs and COPD for adults is limited with most reports evaluating CPs not embedded in the EHR.26-28 One small prospective study among 44 patients hospitalized for COPD exacerbation found that use of a care bundle embedded in the EHR and focused on nursing protocols, medications, and discharge orders was associated with reduced readmission rates, lower LOS, and 90-day hospital costs.37 In our study, readmission rates among pathway users were 16.2% compared to 22% in nonpathway users. While this finding did not reach statistical significance, 16.2% is lower than the reported national average of 19% readmissions among U.S. COPD admissions38 which may suggest some benefit of pathway use on readmissions. Similarly, we found a nonstatistically significant lower LOS and cost among pathway users, but this may have also been driven by a lower proportion of CHF among the pathway cohort compared to the CHF-no pathway group. In bivariate subgroup analyses of the CHF cohort alone, we found the CHF-pathway users had a statistically significant lower LOS (5.3 versus 8.1 days, p < 0.001) and cost ($6098 versus $10,627, p<0.001) as compared to the CHF-no pathway group. A recent systematic review39 analyzed 6 studies published since the year 2000 that met similar criteria for CP intervention. Only one study was conducted in the United States, with other evidence coming from Asia, Australia, and European hospitals. Four of the interventions required a human navigator, no studies addressed EHR integration, and no studies analyzed EHR-based CPs for their effect on process measures such as PR referral. To our knowledge, our study is novel in that regard. Our CPs allow direct ordering from the pathway using links prepopulated with information at the point of care rather than as an extra step clinicians must perform. Seamless EHR integration also makes pathways available at any time, regardless of care setting, such as the ED, medical ward, or intensive care unit. Thus, CPs are immune to changes in doctors, nurses, or case managers during a hospitalization. They are self-contained in that they do not require a nurse navigator or consultant's participation in their deployment, thereby incurring only the cost of developing the pathway.
Our findings are generally consistent with prior literature, suggesting that CPs are effective in decreasing readmission rates, although our readmission finding was not statistically significant. Our finding of a low referral rate of patients for posthospitalization PR is consistent with other studies9,31,40,41 in the United States, which demonstrate rates of PR following COPD hospitalization in the range of 1.5% to 4%. Given the importance of PR in improving patient outcomes, including reducing readmission rates42,43and mortality,31 referral of suitable patients to PR is a critical component of postacute care. A recent analysis of top-performing U.S. hospitals with PR referral rates above the 95th percentile (6.58%) found that systems to identify COPD patients enabled targeted education, care, and discharge planning, including PR referral.44 Our study demonstrates the power of the EHR-embedded clinical pathway to enhance process metrics, such as PR referrals easily and without the need for more people power. The CP quickly redresses any knowledge deficit among providers about both the benefits of and how to order PR, which requires a time-consuming workflow to locate a program.42,45 The pathway prepopulated the link for the PR facility based on the patient’s location and, with one click in the EHR, facilitated referrals. This likely explains our finding of a PR referral rate of 8.5% among the pathway group, which is higher than prior reported PR utilization rates.9,31,40,41
We did not find the same effect of our pathway on tobacco cessation referrals. We believe this is likely attributable both to the paucity of available outpatient tobacco cessation programs and health care professionals’ low knowledge of them. Instead, the pathway directed clinicians and patients to free tobacco cessation resources, including state-funded nicotine replacement. Because referrals and follow-up with these free resources are completed outside of the EHR, we were not able to capture fully the difference in tobacco cessation guidance distributed to patients.
Our finding of a higher odds of discharging to home with pathway utilization is important given the expense of skilled nursing facilities and the dearth of readily available beds, which can lead to increased hospital LOS.46 The pathway nudges providers to anticipate discharge needs on the second day of hospitalization. For example, by measuring ambulatory oxygen saturations and peak inspiratory flow rates early, durable medical equipment can be ordered, and medications can be changed and communicated to the patient well prior to the day of discharge. Early planning for potential needs and support increases the likelihood of a successful home discharge. However, the full mechanisms underlying the higher odds of discharge to home are not clear. Promotion of standards of clinical care, such as use of oral rather than intravenous steroids, found to reduce hyperglycemic episodes,47 and optimized use of other pharmacotherapies, could have played a role. Moreover, the pathway includes a standard protocol to determine home oxygen needs, with streamlined ordering of home oxygen, again prepopulated with information specific to the patient. This may have broadened the scope of providers who could assess this and, in turn, may have expedited the process, rather than relying on physical therapy to document the oxygen needs, which is common practice at our institution. This could also contribute to the trend toward decreased LOS among admissions with pathway use. Adherence to evidence-based practice and decreased LOS could also have contributed to standardized cost reduction.
Our study has some limitations, including our measurement of pathway opening. Although we were not able to locate which element of the pathway was used by the clinician, the fact that there was a difference in Vitamin D ordering suggests that pathways that were “opened” were actively used by clinicians. Another study limitation was the low percentage of relevant hospitalizations for which the COPD pathway was used (14.6%). Encouragement of routine pathway use requires culture change, particularly for common conditions such as COPD, where some health care professionals may believe they do not need clinical guidance for patient management. It is not yet known whether increasing the percentage of hospitalizations during which the COPD pathway is used can have still greater effect on clinical patient outcomes and process metrics such as hospital readmissions, LOS, or health care costs. Also, we were unable to determine which of the 2 pathway components (admission or subsequent day), or subcomponents within these, had an impact on the outcomes assessed. Further, patients did not participate in pathway development. Lastly, there are additional outcomes important for future study, including (but not limited to) the impact of pathway use on evidence-based use of pharmacotherapy, recognition and treatment of ventilatory insufficiency, and recognition of need for palliative care services. The high percentage of patients in our cohort who self-identified as never-smokers was somewhat surprising, but we were unable to formally assess other potential risk factors for COPD as part of this study. While our study focused on COPD, people with other chronic respiratory diseases may benefit similarly from EHR-based CPs. Moreover, while our U.S.-based health care system provides care for a diverse patient population, the generalizability of our findings for patients in other systems, including those with national health care where routine process audit procedures are used (such as for PR referrals), is unknown.
Interpretation
Implementation of an inpatient COPD CP is feasible, and despite a low utilization rate by health care professionals, is associated with improvements in referrals to PR at time of discharge and increased discharges to home. CPs provide a practical vehicle to standardize evidence-based care across a large, complex health care system and ensure that all providers always have access to the most expert knowledge available without the need for additional human resources. As such, they can be powerful platforms to create learning health care systems, and, in turn, have the potential to improve patient outcomes.
Acknowledgments
Author contributions: NK had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. WT performed the primary data analysis and contributed substantially to the data interpretation and writing of the manuscript. NK, DR, and CLR contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Other acknowledgments: The authors would like to acknowledge the time and input of the multidisciplinary COPD Clinical Consensus Group at Yale-New Haven Health System, who contributed substantially to development of the evidence-based content used to develop the Inpatient COPD Care Signature Pathway.
Declarations of Interest
NK, WT, OA, and DR have no conflicts of interest.
CLR serves on COPD Advisory Boards for Sanofi and Regeneron Pharmaceuticals, and has served previously on COPD Advisory Boards for GSK and Boehringer Ingelheim Pharmaceuticals, Inc. She has participated previously in clinical trials relating to COPD funded by AstraZeneca, Inc.