Introduction
In 2020, an international panel of experts introduced the term metabolic dysfunction-associated fatty liver disease (MAFLD) as a replacement for non-alcoholic fatty liver disease (NAFLD).1 This new terminology has since been incorporated into the diagnostic and treatment protocols of the Asian Pacific Association for the Study of the Liver (APASL). While NAFLD is based on exclusion criteria—often criticized for being restrictive, stigmatizing, and clinically impractical—MAFLD adopts positive diagnostic criteria that better reflect metabolic risk and real-world patient profiles. This revised definition addresses the key limitations of NAFLD and enhances clinical applicability.2
MAFLD impacts approximately 38.7% of the global population and is a significant contributor to the development of cirrhosis and hepatocellular carcinoma.3 By 2030, liver-related deaths in the United States are projected to rise by 178%, with an estimated 78,300 fatalities expected by that time. This highlights the growing burden of liver diseases like MAFLD, emphasizing the need for early intervention and treatment.4
Chronic obstructive pulmonary disease (COPD) is 1 of the top 4 causes of death worldwide.5 Although there is recent evidence that the prevalence of fatty liver and liver fibrosis is increasing in COPD patients,6 MAFLD has not been extensively studied in this patient group. Oxidative stress and chronic systemic inflammation, which increase the production of reactive oxygen species and liver inflammation, combined with common risk factors such as aging, smoking, and physical inactivity, are the main mechanisms linking COPD with MAFLD.7
FibroScan, a noninvasive ultrasound technique, is effective in assessing hepatic steatosis and fibrosis. This tool rapidly and accurately quantifies the degree of hepatic steatosis (controlled attenuation parameter [CAP]: S0-S3) and fibrosis (F0-F4). This study utilizes FibroScan to determine the prevalence of hepatic steatosis and fibrosis in patients with COPD and to analyze clinical characteristics between groups with and without MAFLD.
Methods
Inclusion Criteria
Stable COPD patients who visited the asthma-COPD management unit at Nhan Dan Gia Định Hospital in Ho Chi Minh City, Vietnam, between June 2023 and June 2024 and provided their consent to participate in the study were included in this study. Stable COPD patients are defined as those with no significant worsening of symptoms, stable day-to-day symptoms, no significant increase in their COPD Assessment Test (CAT) score, and no rapid decline in lung function (forced expiratory volume in 1 second [FEV1]—only an age-related decrease is expected, without any sharp drop).8
Exclusion Criteria
Patients were excluded from the study if they met any of the following criteria: (1) unable to undergo FibroScan due to the presence of ascites or a body mass index (BMI) over 30 kg/m2, (2) unreliable FibroScan results, indicated by an interquartile range to median ratio greater than 30% or a success rate below 60%, (3) evidence of intrahepatic or extrahepatic cholestasis, or liver congestion caused by heart or lung disease, as determined by abdominal ultrasound prior to FibroScan, (4) acute hepatitis with aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels exceeding 5 times the upper normal limit (35U/L for men, 25U/L for women), and (5) were pregnant or breastfeeding women.
Study Design
This was a cross-sectional, descriptive study.
Sample Size
The sample size was estimated using the formula for estimating the prevalence of MAFLD in COPD patients, with the prevalence from the study by Viglino et al,6 at 41.4%. The minimum sample size required was 93 patients.
Study Methods
The FibroScan 530 machine was used to determine the prevalence and degree of fatty liver. FibroScan, a noninvasive ultrasound technique, is effective in assessing hepatic steatosis and fibrosis. It measures the CAP, which quantifies liver fat content by evaluating the attenuation of ultrasound waves through hepatic tissue. CAP values are reported in decibels per meter (dB/m), with higher values indicating greater degrees of steatosis. CAP thresholds correspond to steatosis grades as follows: S1 (mild steatosis) 234–269dB/m; S2 (moderate steatosis) 270–300dB/m; and S3 (severe steatosis) ≥301dB/m. To diagnose MAFLD, we applied the 2020 criteria of the APASL.9 MAFLD was diagnosed in patients with fatty liver on FibroScan (≥ 5% steatosis, S1 or higher) and who met one of the following 3 criteria9:
- If BMI ≥23kg/m2, MAFLD is diagnosed.
- If the patient has type 2 diabetes, MAFLD is diagnosed.
- If BMI <23kg/m2 and there is no type 2 diabetes, consider the presence of at least 2 of the following metabolic risk factors for diagnosing MAFLD: (a) a waist circumference of >80cm for women or >90cm for men; (b) blood pressure at ≥130/85mmHg or currently on hypertension treatment; (c) plasma triglycerides at ≥150mg/dl (≥1.70mmol/L) or currently on treatment; (d) plasma high-density lipoprotein-cholesterol at <40mg/dl (<1.0mmol/L) for men and <50mg/dl (<1.3mmol/L) for women or currently on treatment; (e) prediabetes (defined as a fasting glucose of 100–125mg/dL [5.6–6.9mmol/L], or 2-hour postprandial glucose of 140–199mg/dl [7.8–11.0mmol/L], or hemoglobin A1c [HbA1c] of [5.7%–6.4%]); (f) a homeostasis model assessment of insulin resistance (HOMA-IR) score of ≥2.5, reflecting insulin resistance; and (g) a plasma high-sensitivity C-reaction protein concentration of > 2mg/L.
Data Analysis
Data were processed using SPSS version 26 (IBM; Armonk, New York). Quantitative variables were expressed as mean±standard deviation if normally distributed, or median and interquartile range if not normally distributed. Differences between 2 means were compared using the unpaired t-test (if normally distributed) or the Mann-Whitney U test (if not normally distributed). Qualitative variables were expressed as frequencies and percentages. Differences between 2 proportions were compared using the Chi-square test or Fisher’s exact test for 2x2 tables where 20% of the cells have an expected frequency <5. A test10 was considered statistically significant when p<0.05. An analysis of the linear correlation between 2 quantitative variables is represented by the correlation coefficient (r) and evaluated using the p-value. If the variables follow a normal distribution, the Pearson correlation is applied; otherwise, Spearman's rank correlation (Rho) is employed for non-normally distributed data. Univariate and multivariable logistic regression were performed to identify factors associated with frequent COPD exacerbations (defined as ≥2 moderate/severe events in the past year). Variables included in the multivariable model were selected based on their clinical relevance and statistical significance (p<0.05) in the univariate analysis.
Ethics Approval
The study was approved by the ethics committee of Nhan Dan Gia Định Hospital, under approval number 79/NDGĐ-HĐĐĐ. Written informed consent was obtained from all patients prior to their participation in the study.
Results
Patient Characteristics
From June 2023 to June 2024, data were collected from 168 COPD patients meeting the inclusion criteria. The mean age was 68.3 ± 8.9 years (range: 43–97), with 89.3% being male. The mean BMI was 21.2 ± 3.5kg/m2, and waist circumference averaged 86.1 ± 11.8cm. Clinical and biochemical characteristics are detailed in Table 1.
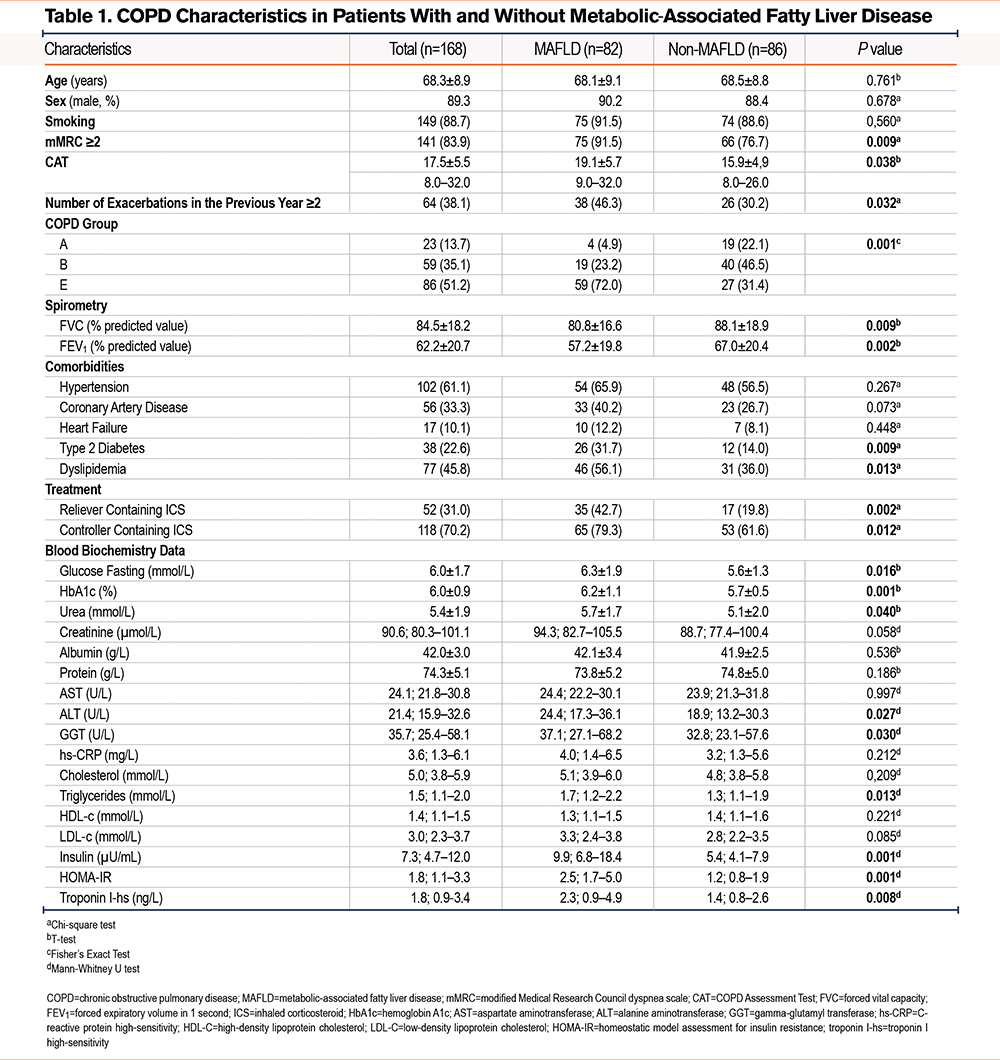
Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease
Of the 168 COPD patients analyzed, 82 (48.8%) were found to have MAFLD. Detailed FibroScan results are presented in Table 2.
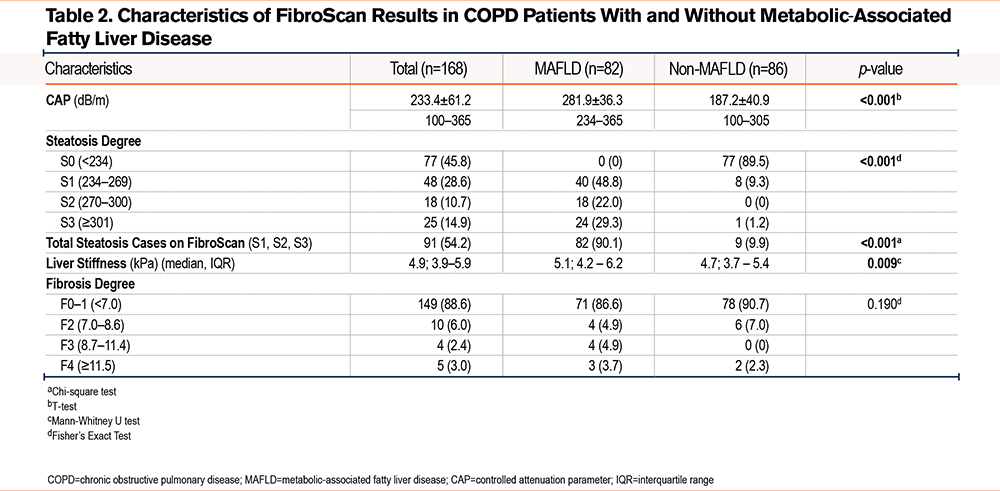
COPD patients with MAFLD compared to those without MAFLD experienced significantly greater dyspnea (modified Medical Research Council dyspnea scale [mMRC] ≥2), had a higher symptom burden (CAT), had a more frequent exacerbation history, and had worse lung function (p<0.05). There was also a higher proportion of patients receiving rescue and maintenance inhaler therapies containing corticosteroids (p<0.05). Patients also demonstrated elevated insulin resistance, blood glucose levels, triglycerides, and troponin I high-sensitivity (p<0.05). COPD patients with MAFLD compared to those without MAFLD showed significantly higher CAP scores, levels of hepatic steatosis, and liver stiffness (p<0.05). Among 168 COPD patients, 91 (54.2%) had hepatic steatosis detected via FibroScan, with the majority (90.1%) meeting diagnostic criteria for MAFLD.
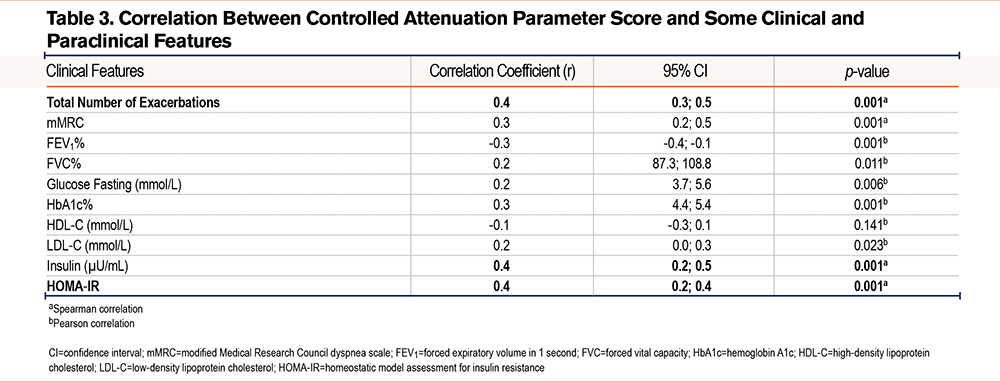
The correlation between controlled attenuation parameter scores and various clinical and biochemical parameters in COPD patients is presented in Table 3. The number of exacerbations and insulin resistance showed a strong correlation with CAP (r>0.33, p<0.05).
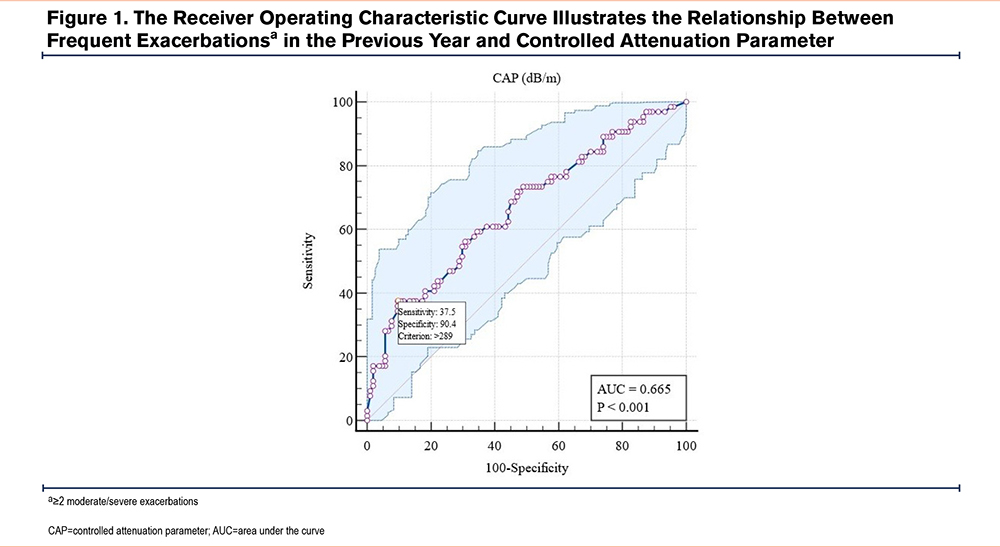
The receiver operating characteristic (ROC) curve illustrates the relationship between frequent exacerbations in the previous year and CAP, which shows an area under the curve (AUC) of 0.665 (p<0.001). At a CAP threshold of >289, the sensitivity is 37.5%, and the specificity is 90.4% (Figure 1).
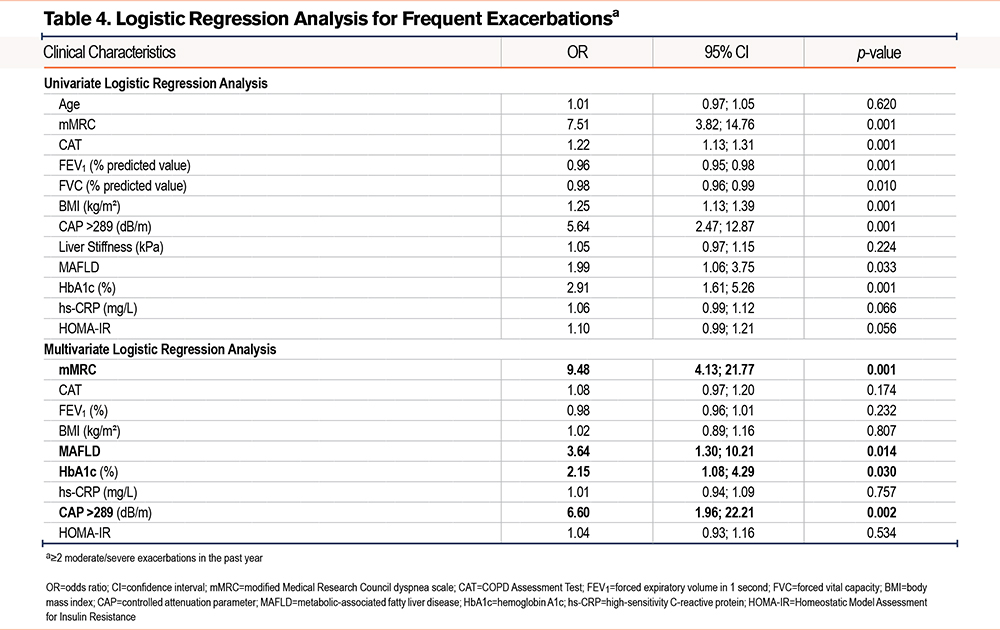
We further investigated the association between frequent exacerbations (≥2 moderate/severe exacerbations in the past year) and some clinical and laboratory characteristics by univariate and multivariate logistic regression (Table 4). Risk factors for frequent COPD exacerbations include a high mMRC score (odds ratio [OR]: 7.51), a high CAT score (OR: 1.22), low FEV1 (OR: 0.96), a high BMI (OR: 1.25), CAP >289dB/m (OR: 5.64), MAFLD (OR: 1.99), and elevated HbA1c (OR: 2.91), all statistically significant (p<0.05). Multivariable logistic regression identified a high mMRC score (OR: 9.48, p=0.001), MAFLD (OR: 3.64, p=0.014), elevated HbA1c (OR: 2.15, p=0.030), and a CAP >289dB/m (OR: 6.60, p=0.002) as independent risk factors for frequent exacerbations (≥2 times/year).
Discussion
Demographic Characteristics
The patients in this study were predominantly male (89.3%) with a mean age of 68.3 years. COPD is more common in males, particularly due to higher historical smoking rates in men, which is reflected here. Waist circumference, which averaged 86.1 cm, is an indicator of central obesity, closely associated with metabolic disorders, insulin resistance, and cardiovascular disease. Hypertension and coronary artery disease were also common comorbidities, although no significant difference was found between the MAFLD and non-MAFLD groups. However, the higher prevalence of cardiovascular diseases in COPD patients may contribute to the severity in these individuals.8,9,11
Prevalence of MAFLD
Among the 168 COPD patients, steatosis was identified using FibroScan in 91 patients (54.2%), defined by a CAP ≥234 dB/m. Of these, 82 patients met the diagnostic criteria for MAFLD according to the 2020 APASL definition. The remaining 9 patients who had steatosis but did not fulfill the MAFLD criteria were classified as non-MAFLD with steatosis. These individuals exhibited hepatic fat accumulation but lacked sufficient metabolic risk factors (e.g., elevated BMI, type 2 diabetes, or at least 2 metabolic criteria) and thus, were not diagnosed with MAFLD.
Nearly half (48.8%) of the COPD patients in the study were diagnosed with MAFLD, highlighting its high prevalence in this population. Diagnosed using FibroScan, MAFLD reflects overlapping metabolic risk factors, including insulin resistance. Advanced steatosis (S3) was observed in 29.3% of MAFLD patients, with 13.5% showing advanced fibrosis (F2-F4). These findings emphasize the need for liver disease screening in COPD patients, especially those with metabolic risk factors. FibroScan results revealed significantly higher CAP scores and liver stiffness in the MAFLD group, indicating more severe steatosis and fibrosis.
Our study found a higher prevalence of MAFLD compared to Vigliano et al’s6 NAFLD rate of 41.4%. This difference may be due to differences in methodologies. Vigliano’s study included only nondrinkers or light drinkers (<20g/day for men, <30g/day for women), used abdominal ultrasound, and excluded other causes of fatty liver while utilizing FibroMax tests (FibroTest, SteatoTest, NashTest) to assess liver conditions. In contrast, our study applied MAFLD criteria, diagnosing fatty liver via FibroScan alongside metabolic disorders without excluding alcohol consumption or other liver diseases. MAFLD’s "positive criteria" approach led to a higher prevalence, capturing more patients. This finding underscores the need for increased attention to MAFLD in COPD patients, given its significant overlap with metabolic dysfunction and potential liver complications.
Miao et al's study12 reported a prevalence of MAFLD at 20.4% (n=519) and NAFLD at 18.4% (n=469) among middle-aged individuals from 25 communities across 4 cities in China in 2020. These rates are lower than those found in our study, suggesting that the COPD and MAFLD patient group not only shares common risk factors such as chronic inflammation, oxidative stress, sedentary lifestyle, and smoking but also experiences a synergistic effect, increasing disease prevalence compared with the general population in Miao et al’s report. This finding introduces a new perspective for managing COPD patients with MAFLD, emphasizing the need for better treatment and control of metabolic factors.
COPD Characteristics
Smoking remains the most significant risk factor for COPD, and this study supports this, with 88.7% of patients being smokers. Both the mMRC scale and CAT scores were higher in the MAFLD group, reflecting more severe dyspnea and worse health status. Patients with MAFLD experienced more limitations in daily activities due to worse lung function than the non-MAFLD group.
The frequency of exacerbations in the previous year was higher in MAFLD patients (46.3% had ≥2 exacerbations versus 30.2% in non-MAFLD, p=0.032). Exacerbations in COPD are critical events that often lead to hospitalizations and further lung function decline. The link between MAFLD and higher exacerbation rates suggests a potential interaction between liver dysfunction, systemic inflammation, and lung health. Lung function was significantly worse in the MAFLD group, with reduced FEV1, forced vital capacity, and peak expiratory flow.
Blood Biochemistry
Glucose fasting and HbA1c levels were significantly higher in the MAFLD group, reflecting poorer glycemic control. Elevated glucose and HbA1c levels are associated with both MAFLD and COPD, indicating an increased risk of complications such as cardiovascular disease and exacerbations. Although ALT and gamma-glutamyl transferase levels were higher in the MAFLD group, the median values for AST, albumin, and total protein were within the normal range. This may suggest that while liver steatosis is common in COPD patients, overt liver dysfunction is apparently unaffected.
High-sensitivity C-reactive protein (hs-CRP), a marker of systemic inflammation, was higher in MAFLD patients, though the difference was not statistically significant (p=0.212). Chronic inflammation is a hallmark of both COPD and MAFLD, and elevated CRP levels may indicate a shared inflammatory pathway driving both diseases.
Correlation Between Controlled Attenuation Parameter and Clinical, Biochemical Parameters in COPD Patients
The results of linear regression analysis revealed several notable points: the degree of hepatic steatosis was strongly correlated with key clinical and biochemical indices such as the frequency of COPD exacerbations, mMRC, HbA1c, and metabolic indices like triglycerides and HOMA-IR. These indices are closely related to the metabolic and chronic inflammatory status of COPD patients with hepatic steatosis, suggesting that liver fat accumulation may play a significant role in exacerbating COPD. Specifically, the positive correlation between CAP and the number of COPD exacerbations (r=0.4, p<0.001) and CAP with mMRC (r=0.3, p<0.001) indicates that higher levels of hepatic steatosis may lead to poorer respiratory status and increased risk of exacerbation. Additionally, higher HbA1c levels in patients with elevated CAP (r=0.3, p<0.001) further emphasize the association between hepatic steatosis and glucose metabolism disorders, a potential risk factor that worsens COPD. Conversely, CAP was negatively correlated with the percentage of FEV1 (r=-0.3, p=0.001), suggesting that the more liver fat present, the lower the patient’s FEV1, the more severe the airway obstruction according to Global Initiative for Chronic Obstructive Lung Disease8 criteria.
The study by Viglino et al6 found that COPD patients with NAFLD tended to have worse metabolic indices, but did not specifically analyze the correlation between CAP and clinical indices, indicating that our study extends this analysis with more detailed results. Moreover, in Viglino et al’s study,6 the correlation between CAP and exacerbation frequency was not analyzed, which is a contribution of this study in enhancing the understanding of the impact of hepatic steatosis on COPD.
Miao et al12 found a similar correlation between hepatic steatosis and metabolic factors such as triglycerides and HbA1c, but they did not focus on the COPD patient group, instead on the general population. Our study delves into the specific relationship between COPD and hepatic steatosis, emphasizing that metabolic factors not only affect liver function but also have a negative impact on respiratory status and exacerbation risk. Another study evaluating CAP in NAFLD patients by Hu et al13 showed that CAP was closely linked to metabolic factors such as BMI, HbA1c, and triglycerides, similar to our findings. However, Hu et al’s study did not focus on the specific group of COPD patients.
Figure 1 illustrates the ROC curve between CAP and the frequency of exacerbations of COPD, with an AUC of 0.665 (p<0.001). At a CAP threshold of >289dB/m, sensitivity was 37.5% and specificity was 90.4%. This suggests that CAP, a measure of fatty liver, is a good predictive tool for the risk of COPD exacerbations, especially when CAP exceeds the 289dB/m threshold. Although sensitivity is not very high, the high specificity makes CAP a fairly accurate tool for predicting high-risk COPD patients.
Table 4 presents univariate regression analysis to identify clinical and biochemical factors associated with frequent exacerbations. The results showed several statistically significant factors: higher mMRC scores were strongly associated with frequency of exacerbation (OR: 7.51, p=0.001). CAP > 289dB/m (OR: 5.64, p=0.001) and MAFLD (OR: 1.99, p=0.033) were also significant risk factors, indicating that hepatic steatosis and higher liver fat content may increase exacerbation risk in the previous year. Other metabolic indices such as HbA1c and BMI were also associated with increased exacerbation risk, with ORs of 2.91 and 1.25, respectively. These findings highlight the important role of metabolic dysfunction-related hepatic steatosis in the clinical risk of exacerbations.
Table 4 expands the analysis with a multivariate regression model, adjusting for potential risk factors. The results showed that the following factors remained independently statistically significant: high mMRC (OR: 9.48, p=0.001) was the strongest risk factor associated with exacerbation frequency. This is consistent with previous studies on the role of dyspnea in predicting COPD exacerbations. MAFLD remained significant with an OR of 3.64, p=0.014, indicating that patients with metabolic dysfunction-related hepatic steatosis are more susceptible to COPD exacerbations. HbA1c was also independently associated with exacerbation frequency, underscoring the role of glucose control in managing COPD. These findings suggest that comprehensive management of metabolic factors, particularly hepatic steatosis, could help reduce the risk of exacerbations in COPD patients.
According to Viglino et al,6 metabolic dysfunction-related factors were also identified as important risks for COPD, but detailed analysis of the relationship between CAP and exacerbations was lacking. Our study has highlighted the role of CAP as well as MAFLD as risk factors for increasing the frequency of COPD exacerbations in the previous year. This suggests that COPD and MAFLD may interact with each other to increase the risk of exacerbations, opening a new research direction for the simultaneous management of metabolic and respiratory diseases.
Specifically, the study’s results highlighted how MAFLD might play a unique and synergistic role in COPD through shared mechanisms such as chronic systemic inflammation, oxidative stress, insulin resistance, and metabolic dysregulation, which were not equally present in other cardiometabolic comorbidities like hypertension or coronary artery disease. Recent studies, including Tsutsumi et al,11 have also emphasized the independent association between MAFLD and COPD, even after adjusting for age and smoking, suggesting a potentially more direct interaction compared to other metabolic conditions. Moreover, in our final multivariable logistic regression model, the independent risk factors for frequent COPD exacerbations were degree of dyspnea (mMRC), presence of MAFLD, elevated HbA1c, and CAP >289dB/m. This pattern suggests that hepatic steatosis and metabolic dysfunction, particularly in the form of MAFLD, may have a stronger impact on COPD exacerbation risk than other common cardiometabolic comorbidities. These findings support the hypothesis that MAFLD is not merely a coexisting condition but may play an active role in worsening COPD outcomes, possibly through systemic metabolic-inflammatory pathways.
Although the ROC analysis demonstrated a statistically significant association between CAP and frequent exacerbations, with a threshold of >289dB/m, the AUC was 0.665, indicating only moderate discriminative power. Therefore, CAP should not be viewed as a strong standalone predictor. However, its high specificity (90.4%) suggests value in identifying a subgroup of patients at particularly high risk. Taken together, these results emphasize that CAP and MAFLD are best interpreted within a broader clinical and metabolic context, alongside established predictors such as mMRC and HbA1c, to more accurately stratify exacerbation risk in COPD patients.
Limitations
This study has some limitations. First, its cross-sectional design restricts the ability to establish causal relationships between MAFLD and COPD exacerbations. While associations were observed, it remains unclear whether MAFLD contributes to increased exacerbations or is simply a comorbid condition resulting from shared risk factors. Longitudinal studies with follow-up data are needed to better understand the long-term clinical outcomes of COPD patients with coexisting MAFLD. Second, the cohort consisted predominantly of male patients (89.3%), limiting the generalizability of the findings to female COPD patients. The gender imbalance reflects the smoking patterns in the studied population but remains a potential source of bias.
Conclusion
Our study demonstrates a high prevalence of MAFLD in COPD patients (48.8%). MAFLD is associated with worse lung function and an increased frequency of exacerbations of COPD. Elevated CAP scores (>289dB/m) were found to be a strong predictor of frequent exacerbations. The study also shows that MAFLD is an independent factor that increases the risk of exacerbations in COPD patients, further highlighting the connection between liver fat accumulation and respiratory outcomes.
Clinical Implications
COPD patients should be screened for MAFLD; those with coexisting MAFLD should be closely monitored and given tailored management due to a higher risk of exacerbations, especially with severe dyspnea, high HbA1c, and CAP > 289dB/m.
Acknowledgments
Author contributions: DH, TT, and LV conceived and designed the study. DH, TH, and AH handled data collection. DH and DT performed the analysis and interpretation of the results. DH drafted the manuscript. TD, LH, VC, and NH provided data collection and administrative management support. All authors reviewed the results and approved the final version of the manuscript.
Other acknowledgments: We extend our sincere thanks to the patients and colleagues at Gia Dinh People’s Hospital for their invaluable support, which made the successful completion of this study possible.
Declaration of Interest
The authors declare that they have no potential conflicts of interest.