Running Head: Asthma, COPD, and Asthma-COPD Overlap Syndrome
Funding Support: Supported by R01HL161674 (PI: JGZ).
Date of Acceptance: June 20, 2025 | Published Online: June 23, 2025
Abbreviations: ACO=asthma-COPD overlap; AHRQ=Agency for Healthcare Research and Quality; AKI=acute kidney injury; BMI=body mass index; CHF=congestive heart failure; COPD=chronic obstructive pulmonary disease; HCUP=Healthcare Cost and Utilization Project; HTN=hypertension; ICD-9-CM=International Classification of Diseases, Ninth Revision, Clinical Modification; IQR=interquartile range; IRB=institutional review board; LOS=length of stay; NDX=number of diagnoses at discharge; NRD=Nationwide Readmissions Database; OR=odds ratio; PPV=positive predictive value; SE=standard error
Citation: Modak M, Rowlands WM, Sleiman J, Attaway AH, Bleecker ER, Zein J. Hospitalization outcomes of patients with asthma, COPD, and asthma-COPD overlap syndrome. Chronic Obstr Pulm Dis. 2025; 12(4): 260-273. doi: http://doi.org/10.15326/jcopdf.2024.0566
Online Supplemental Material: Read Online Supplemental Material (296KB)
Background
Among respiratory diseases, asthma and chronic obstructive pulmonary disease (COPD) are the most common in the United States, individually accounting for a health care cost of over $34 billion annually.1,2 Each diagnosis is associated with significant morbidity; the co-occurrence of asthma and COPD being noted to have a particularly negative effect on patients’ quality of life and health care utilization.3,4 These patients display clinical features of both asthma and COPD with varying degrees of reversible airflow limitation and are described by the term asthma-COPD overlap (ACO). Despite being first proposed as an entity in 2015, ACO still does not have specific diagnostic criteria of its own and encompasses several heterogeneous presentations along the spectrum of obstructive lung disease.5
In comparison to COPD, ACO has been associated with a younger age and higher body mass index (BMI), but not with a male gender predominance.6 The ACO phenotype is associated with more severe airflow limitation and more pronounced bronchial hyperresponsiveness when compared to either COPD or asthma individually,7-10 and patients with ACO frequently report higher disease severity3 and poorer health-related quality of life.6,7 These findings are not consistent across all studies,11 and this variation may be related to differences in selection or diagnostic criteria.6 In respect to health care metrics, ACO has been associated with higher rates of exacerbations, hospitalizations, and emergency department visits,3,6-8,12 all of which translate into increased disease burden and higher health care costs.3,13
Interestingly, despite these prevalent reports on increased morbidity with ACO compared to asthma or COPD alone,3,6 the diagnosis does not consistently demonstrate increased mortality.14,15 In prior work, ACO was noted to carry higher mortality than asthma alone, but lower or similar mortality to COPD alone.14-16 To date, the effect of the ACO phenotype on hospitalization outcomes, such as hospital mortality, hospital length of stay (LOS), hospitalization-related complications, or readmission rate, have not been well described. In this study, we use a large administrative public database to test the hypothesis that the ACO phenotype is associated with worse hospital outcomes compared to asthma or COPD alone.
Methods
Study Dataset
We analyzed data from the Healthcare Cost and Utilization Project (HCUP) Nationwide Readmissions Database (NRD). Developed through a federal-state-industry partnership and sponsored by the Agency for Healthcare Research and Quality (AHRQ), HCUP includes the largest collection of administrative longitudinal hospital care data in the United States, with all-payer, encounter-level information.17 Admissions data from January 2012–December 2015 were included for the consistency of using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis in the database. As the protocol used only publicly available deidentified data, it did not meet criteria for institutional review board (IRB) approval and formal review was waived by the Mayo Clinic IRB. Consent for participation was not applicable.
Study Population
ICD-9-CM codes were used to identify patients from the NRD with a principal diagnosis at index admission of asthma (ICD-9-CM:493), COPD (ICD-9-CM: 491,492,496), or ACO.17 In the absence of a specific ICD-9-CM code, patients with concurrent diagnoses of COPD and asthma on index admission were designated as the ACO population.6 Current smokers were identified using ICD-9-CM = 305.1 (active nicotine addiction), and past smokers with ICD-9-CM = V15.82 (a history of tobacco). The index admission was defined as the first admission associated with any of these diagnoses. Patients were excluded from the cohort if they were 18 years or younger, if they had aspiration pneumonia, or if they had any chronic pulmonary conditions other than asthma or COPD that could confound outcomes. Patients who were discharged from their index admission in December 2015 and those who died during index admission were also excluded for the analysis of 30-day readmissions.17
Data Collection and Statistical Analysis
The dataset retrieved from HCUP included information on patients’ demographic characteristics, socioeconomic status (estimated household income, primary insurance type), and medical comorbidities (identified using ICD-9-CM codes). Included metrics of hospitalization outcomes were hospital LOS, mortality, 30-day all-cause readmission rate, and asthma-related readmission rate (for patients with a diagnosis of asthma or ACO), as well as total charge of the hospitalization (at index admission and readmission). Mortality was determined for each admission type and diagnosis by the percentage of patients with a HCUP discharge disposition of “20,” indicating in-hospital death. Lastly, both respiratory and nonrespiratory complications during hospitalization (respiratory failure, sepsis, shock, acute kidney injury [AKI], delirium, encephalopathy, and stroke) were included. Unfortunately, no medication or outpatient clinical data were available.18
These data were collected and analyzed for the index admission as well as 30-day asthma-related readmissions and all-cause readmissions. We used the HCUP Cost-to-Charge Ratio Files to translate hospital charges into actual costs inflated to 2015 U.S. dollars using the Gross Domestic Product price index.17,19 To predict national estimates and compute descriptive statistics, including medians (interquartile range[IQR]) and percentages, we used the analytic sample weights (discharge-level weight) according to published HCUP-NRD guidelines (HCUP Methods Series Calculating NRD Variances).18
None of the continuous variables were found to be normally distributed using a quantile-quantile plot analysis. Three group (asthma, COPD, and ACO) comparisons were performed using the Kruskal-Wallis rank test, and categorical variables were compared using a χ2 test. The impact of having asthma, COPD, or ACO on hospitalization outcomes was evaluated using weighted regression analysis. We used weighted logistic regression for binary outcomes such as respiratory failure, sepsis, shock, AKI, encephalopathy, stroke, in-hospital death, and 30-day readmission, presenting the results as weighted odds ratios (ORs) with 95% confidence interval (CI). Similarly, we used weighted linear regression and a 95% CI to analyze continuous outcomes such as LOS and costs, which were log-transformed, as well as the exponential of the slope parameter, which reflects the relative change in each outcome.17 All adjusted models included age, sex, primary insurance type, predicted median household income per ZIP code, obesity, diabetes, congestive heart failure, depression, hypertension, smoking history, alcohol use, anemia, rheumatologic disorders, congestive heart failure, valvular heart disease, coagulation disorders, hypothyroidism, chronic liver disorders, electrolytes imbalance, cancer, lymphoproliferative disorders, renal failure, and hospital characteristics. Race was not included in the dataset and, therefore, not adjusted for. All tests were 2-tailed and performed at a significance level of .05. The Bonferroni method was used to correct for multiple comparisons. Analyses were performed using the SURVEY package in R (R Foundation for Statistical Computing; Vienna, Austria).
Sensitivity Analysis
A sensitivity analysis was performed by stratifying COPD into chronic bronchitis and emphysema to account for clinical and outcome differences between the 2 conditions (Table S1 in the online supplement).
Results
Patient Characteristics at Time of Index Admission
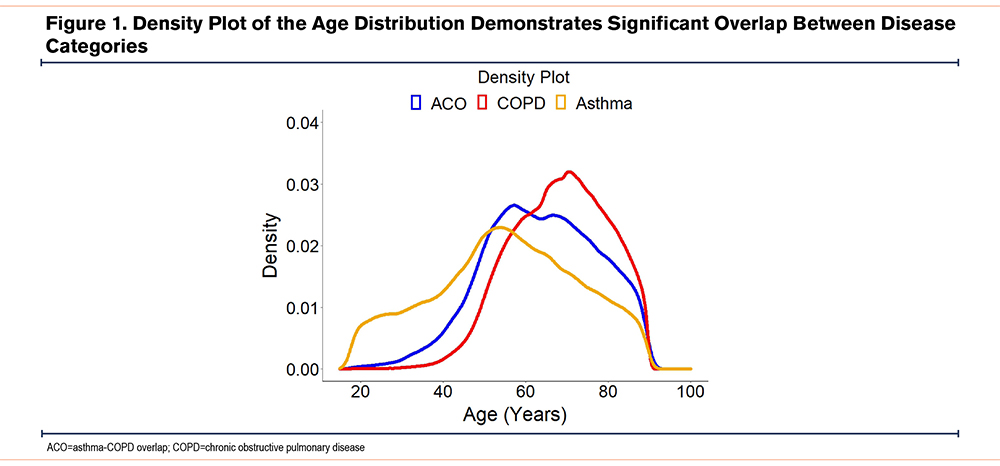
A total of 2,522,013 patients met the inclusion criteria and were included in the final analysis. Of those, 1,732,946 (68%) had COPD, 668,867 (26.5%) had asthma, and 120,200 (4.8%) were designated as having ACO as they carried the ICD-9-CM codes of both asthma and COPD. Patients with ACO were older (median [IQR]: 63 [74; 54]) than those with asthma (median [IQR]: 56 [68; 45]), but younger than those with COPD (median [IQR]: 69 [77; 60]) (p<0.001), with the full distribution of ages depicted in Figure 1. The proportion of current smokers older than 25 years was notably higher in ACO (27.2%) as compared to asthma (24.3%), but lower than those with COPD (35.7%) (p<0.001). Likewise, the proportion of past smokers older than 25 years was higher in ACO (21.4%) as compared to asthma (17.7%), but lower than those with COPD (24.9%) (p<0.001). More patients with ACO (69.9%) and asthma (71.6%) were female compared to those with COPD (54.7%) (p<0.001). Baseline characteristics of each study cohort at index admission are displayed in Table 1.
Comorbidities, Disease Severity, and Mortality Risk
Patients with ACO were more likely to have multiple age-related comorbidities than those with asthma or with COPD. Except for comorbid congestive heart failure, patients with ACO had higher rates of obesity (26.8%), diabetes (34.3%), hypertension (67.7%), and depression (18.7%) when compared to those with asthma or COPD (p<0.001) (Table 1). Patients with COPD had the highest All Patient Refined Diagnosis-Related Group (class 3 or 4) severity20 and mortality risk (40.1% and 32.3% respectively), followed by patients with ACO (36.6% and 24.9% respectively), and lastly by patients with asthma (26.1% and 17.6% respectively).
Characteristics of Index Admissions
Relative to both COPD and asthma, ACO was associated with increased hospital LOS. The median [IQR] LOS at index admission was 4 [6, 2] days for ACO versus 3 [5, 2] days for both COPD and asthma (p<0.001). In addition, index admissions with ACO were associated with the highest cost of care ($6282 [$9711; $4223]), followed by COPD ($6082 [$9489; $4081]) and asthma ($5422 [$8511; $3564)] (p<0.001). However, the coexistence of asthma and COPD did not lead to higher mortality. In fact, mortality at index admission was the highest among patients with COPD (2%), followed by those with asthma (0.85%), and was least in patients with ACO (0.7%) (p<0.001) (Table 2). Adjusted analysis demonstrated that hospital LOS and cost were lower for patients with COPD and asthma compared to ACO. In contrast, mortality was higher in patients with COPD (OR [95%]: 2.104 [1.844; 2.401]), and asthma (OR [95%]: 1.592 [1.382; 1.832]) as compared to ACO (p<0.001) (Table 3).
Characteristics of 30-Day All-Cause and Asthma-Related Readmissions
Readmission rate, cost, and mortality were highest for patients with COPD. All-cause readmission rate in the ACO group (11.5%) was slightly higher than asthma (10.7%) but still lower than the COPD group (15.7%) (p<0.001). All 3 cohort groups had a median time to readmission of 12 days and an LOS of 4 days (Table 2). The cost of stay at readmission was, however, significantly higher for COPD (median [IQR]: $8091 [$13,975; $5016] U.S. dollars) than the ACO group (median [IQR]: $7652 [$13,096; $4778] U.S. dollars), with both being higher than asthma (median [IQR]: $7167 [$12,370; $4425]) (p<0.001) (Table 2). Among patients readmitted within 30 days of index hospitalization, mortality was highest in patients with COPD (5.1%), followed by ACO (3%) and asthma (2.2%) (p<0.001).
Adjusted analysis demonstrated that hospital cost and LOS were higher in patients with COPD as compared to ACO, but not in those with asthma. Similarly, hospital mortality was higher in patients with COPD as compared to ACO (OR [95%]: 1.386 [1.175; 1.634]) and lowest in those with asthma (OR [95%]: 0.897 [0.747; 1.077]) (Table 3). The hospital LOS, number of days to asthma-related readmission, and hospital mortality did not differ significantly between the asthma group and the ACO group (Table 2). Similar results were seen after adjusted analysis (Table 3).
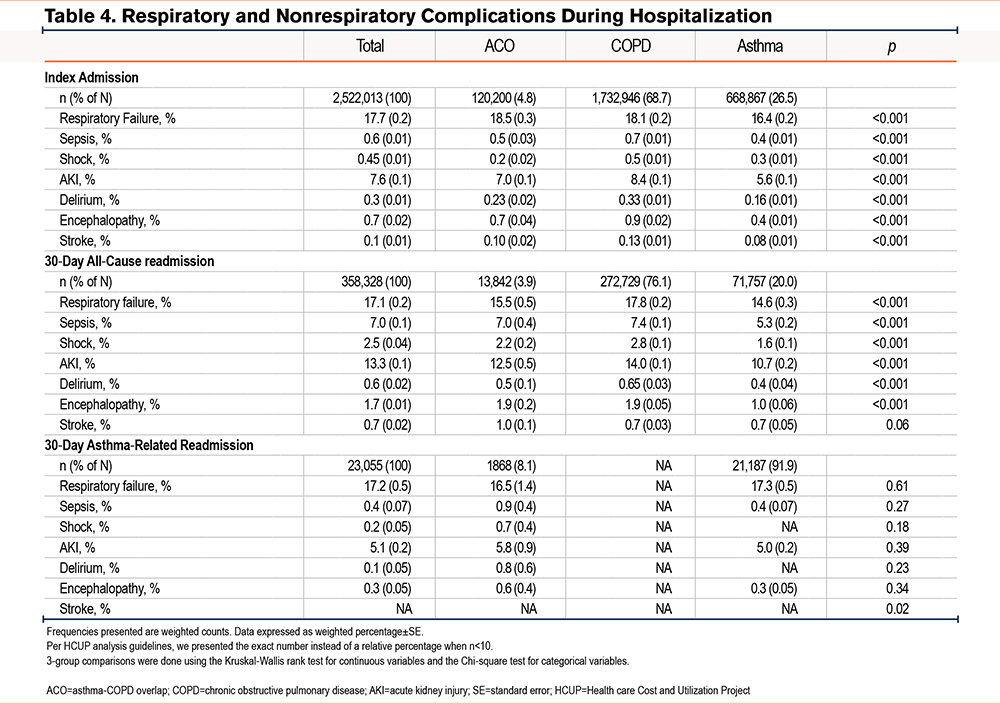
Respiratory and Nonrespiratory Complications
During index admission, the rate of respiratory failure was the highest in patients with ACO (18.5%) compared to those with COPD (18.1%) and asthma (16.4%) (p<0.001). However, the rates of other hospital complications, such as sepsis, shock, AKI, delirium, and encephalopathy, were highest among patients with COPD (Table 4). In contrast to the index admission, respiratory failure during 30-day all-cause readmissions were lower in ACO versus COPD (15.5 versus 17.8% respectively). Hospital complications during 30-day all-cause readmissions were lowest in the asthma group and, apart from stroke, highest in the COPD group (Table 4). During asthma-related readmissions, the rate of respiratory and nonrespiratory complications did not differ significantly between patients with ACO and those with asthma alone (Table 4).
COPD Subgroup Sensitivity Analysis
Among patients with COPD, 1,561,520 (90.1%) carried an ICD-9-CM code for chronic bronchitis and 171,425 (9.9%) carried an ICD-9-CM code for emphysema (Table S1 in the online supplement). As compared to patients with chronic bronchitis, those with emphysema were younger (median [IQR] age: 68 [77; 59] versus 69 [77; 60], p<0.001), and were more likely to be females (52.2% versus 55.0%, p<0.001) (Table S2 in the online supplement). Other differences between these 2 groups are listed in Supplementary Table 2 in the online supplement. Compared to patients with ACO, the risk of hospital mortality during index admission was elevated among individuals with chronic bronchitis (adjusted OR [95% CI]: 1.43 [1.26; 1.64]) but was highest among those with emphysema (adjusted OR [95% CI]: 7.63 [6.65; 8.75]). The hospital cost was higher among patients with emphysema (adjusted OR [95% CI]: 1.06 [1.05; 1.07]) but lower among patients with chronic bronchitis (adjusted OR [95% CI]: 0.94 [0.93,0.94]) when compared to those with ACO (Table S3 in the online supplement). However, the hospital LOS was lower among both patients with emphysema (adjusted OR [95%CI]:0.91 [0.90; 0.92]) and chronic bronchitis (adjusted OR [95% CI]: 0.92 [0.91; 0.93]) when compared to those with ACO (Table S4 in the online supplement).
Discussion
Our study shows that despite higher rates of age-related comorbidities, increased length of stay, and higher health care costs, ACO was not associated with higher mortality compared to asthma or COPD alone during index admissions. Additionally, in contrast to our hypothesis, this increased baseline morbidity did not translate into higher rates of in-hospital nonrespiratory complications, risk for readmission, or mortality when compared to COPD.
Similar to prior studies, the ACO population had higher rates of congestive heart failure, diabetes, and hypertension when compared to the asthma population.21 Notably, the prevalence of obesity in ACO patients was similar to that seen in asthmatics but was significantly higher when compared to COPD patients. Obesity has been previously correlated with worse lung function and outcomes in asthmatics, thought to be in the setting of elevated systemic inflammation and airflow limitation,22,23 and patients with ACO are noted to have high levels of interleukin-6,10,24 an inflammatory marker linked to the development of coronary artery disease, obesity, and diabetes,25 all of which implicates a metabolic syndrome-like process in the pathogenesis of more severe obstructive pulmonary disease. With demonstrated higher exacerbation rates, health care utilization, and costs21,24 ostensibly leading to recurrent systemic steroids exposure,13,26 patients with ACO may further exacerbate this underlying syndrome, resulting in increased morbidity. A comparable constellation of characteristics was observed in the National Heart, Lung, and Blood Institute-sponsored Severe Asthma Research Program severe asthma phenotype cluster analysis,27 where they identified a group of severe asthmatics with low reversibility and persistent airflow limitation despite significant airway reactivity, likely due to high levels of neutrophilic involvement, type-2 inflammation, and resulting airway remodeling over a lengthy duration of disease. This cluster closely mirrored our ACO population in being older, with more comorbidities and health care utilization.
The higher costs and hospital LOS observed in the ACO population during the index admission, as compared to either asthma and COPD, is likely related to the higher rates of respiratory failure and comorbidities, as seen previously in Kumbare et al.28 These findings were discordant with the lower index admission mortality rate of patients with ACO, but it should be noted that patients with asthma who died during the index admission had a median lower LOS (5 days versus 7) and lower cost of admission ( $15,966 versus $17,773) as compared to patients with ACO, which could contribute to this discrepancy. There were also differences with discharge disposition, such as patients with ACO being more likely than patients with asthma to be discharged to a rehabilitation facility (0.7% versus 0.1%) and less likely to have a routine discharge to home (76.3% versus 82.8%), indicating increased frailty with the ACO group that would put them at a higher risk of death in the posthospitalization period. Due to the limitations of our dataset, we were not able to capture these postdischarge deaths and thus, may have found a higher mortality in the asthma group. In contrast to the index admissions, ACO was found to have higher mortality during 30-day all-cause and asthma-related readmissions when compared to asthma. Patients with ACO also had a higher rate of readmission when compared to the asthma population (11.5% versus 10.7%), both of which underscore the worse clinical outcomes in ACO, even within only a 30-day window. Previous studies that demonstrated increased mortality in ACO when compared to asthma included both inpatient and outpatient data over a longer time period,16 and our observed differences in mortality likely would be more pronounced if a different time cut-point (90 days versus 30 days) was chosen.10,24,29
Compared to the index admission, patients with ACO had lower rates of respiratory failure than patients with COPD during readmission. These higher rates of respiratory and nonrespiratory in-hospital complications and mortality in the COPD population were reflected in the higher costs associated with their readmissions. Of note, these findings were likely influenced by the high proportion of patients with chronic bronchitis within the COPD cohort. In fact, once COPD was stratified into the subsets of chronic bronchitis and emphysema, there was a significant difference in outcomes, which highlights the effect of disease subphenotypes. When assessing asthma-related admissions, there were no differences in hospital complications between patients with ACO as compared to patients with asthma. However, there were significant differences in hospital complications between the 2 groups when looking at all-cause readmissions. This implies that there may be multiple phenotypes of patients within our ACO subgroup with different burdens of disease, similar to our COPD cohort, due in part to the heterogeneity in inflammation underlying these obstructive diseases.30-32
Though there was significant overlap between the IQR of ages in the study populations, patients with COPD were the oldest, conferring a higher likelihood of hospitalization complications independent of their pulmonary disease, as well as presumed higher lifetime cumulative doses of corticosteroid use and cachexia (reflected by lower BMI), all of which likely contributed to having the highest mortality during index admission and 30-day all-cause readmission. While we adjusted for age in our multivariable regression models, we agree that we cannot completely account for the bias introduced in clinical practice, where clinicians have a tendency to label younger adults with obstruction as asthmatics and older individuals as having COPD. However, these results are consistent with previously noted rates of inpatient mortality associated with COPD in Japan,26 and suggest increased disease burden and severity when compared to the ACO and asthma populations.
When comparing the baseline attributes of the study populations, we were able to identify several characteristics that set the ACO cohort apart from the asthma and COPD cohorts as a group of participants with a distinct relationship of comorbidities and mortality. While the ACO and COPD populations had a similar initial rise in their density plot curve (Figure 1), the median age of patients with ACO was significantly less than that of those with COPD, a characteristic of this population which has been previously noted.6 This implies that individuals with ACO had symptomatic obstructive airway disease at an earlier age than COPD patients, and that ACO may represent a unique phenotype with overlapping clinical features between the early reversible obstruction of asthma and the late fixed obstruction of COPD33 on a spectrum of common disease, as suggested previously in the Dutch hypothesis.34 Along these lines, the differences in the comorbidity profiles and mortality rates seen in our analysis, combined with previously detailed differences in exacerbation rates and hospital utilization, also suggest that ACO is a separate obstructive disease entity from asthma and COPD24 with its own disease course. This distinction is pertinent given the shift from a dichotomy of obstructive airway disease to multiple endotypes.
Limitations
A major limitation of our analysis was the dependence on diagnostic codes for the delineation of our study groups, due to the use of an administrative database without other corroborating clinical data to confirm appropriate patient assignation. However, we feel that the utilization of diagnosis coding alone was appropriate since this standard has been widely employed in other epidemiologic research,13,35 as well as by the National Committee for Quality Assurance and the AHRQ to guide quality measures. The use of asthma ICD-9 codes alone has been studied previously36,37 when compared to the accuracy of medical record review and found to have a positive predictive value (PPV) of 75%–85%. A similar analysis38 looking at identifying COPD admissions employed the use of multiple diagnostic codes for COPD with a PPV of 85.6%. It is acknowledged that the use of administrative data as the sole classifier in each study was noted to underrepresent the true populations with each condition, with accompanying medical or pharmacologic data being the primary driver of improved accuracy. In the future, a study comparing similar groups with the addition of biomarkers like interleukin-6 as well as lung function markers, such as the forced expiratory volume in 1 minute or diffusing capacity of the lung, would help both validate cohort selection, and possibly support a clearer distinction between late stage obstructive asthma and true ACO, as has been described previously.10
While the HCUP database enabled us to minimize bias by analyzing a large, nationally representative sample of patients, the inpatient-only dataset and a short follow-up period of 30 days likely self selected patients with disproportionately severe comorbidities and poor access to primary care (more emergency department utilization). This dependence on hospitalization data, as well as the limited time period of 3 years, may have introduced an element of selection bias, as many patients with these chronic diseases are managed well in the outpatient setting for years, and we were not able to capture their outcomes or any out-of-hospital deaths. Furthermore, the dataset lacked information on biological data such as laboratory values and other important granular data. For example, quantitative smoking history, pulmonary function testing, exacerbation history, dyspnea scales, exercise capacity, and prior therapies in these 3 groups, which can all be used to classify airway disease, were not included. Hence, we were unable to closely compare our work with prior longitudinal studies in which patients were diagnosed on the basis of spirometric criteria. With a decline in lung function being associated with worse outcomes in COPD, it would be interesting to see if the same relationship can be seen in ACO.39 Additionally, due to only using hospitalization data, we could not determine the initial hospitalization risk for patients with each disease in the general population. Despite such limitations, our study was able to identify several trends in the comorbidity profiles and hospital outcomes when observing the asthma, COPD, and ACO populations.
Conclusion
Compared to either asthma or COPD, ACO was associated with increased health care costs and length of stay during index admissions. However, this did not translate into a higher readmission rate or mortality, both of which were highest in the COPD cohort. Apart from mortality, there were also significant differences in comorbidities and complications between COPD, asthma, and ACO, as well as between subgroups of ACO itself, which underscores the heterogeneity of the different airway diseases. Further studies correlating biological data (including patterns of inflammation), demographic characteristics, and access to health care with hospitalization outcomes are needed to better understand these findings and their implications.
Acknowledgments
Author contributions: MM, WMR, and JGZ made substantial contributions to the conception or design of the work. JGZ acquired, analyzed, and interpreted the data for the work. MM, WMR, JS, AHA, JGZ, and ERB contributed to drafting the work or revising it critically for important intellectual content. JGZ agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MM, WMR, JS, AHA, JGZ, and ERB gave the final approval of the version to be published.
Data sharing statement: The data used in this study are openly available for fee-based use through the Agency for Healthcare Research and Quality, with whom JGZ signed a data user agreement.
Declaration of Interest
The authors declare that they have no competing interests.