Running Head: Clinical Control of COPD in Portugal
Funding Support: None
Date of Acceptance: July 15, 2025 | Published Online: July 22, 2025
Abbreviations: BD=bronchodilator; CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; CTR=current treatment regimen; DLCO_SB%=diffusing capacity of the lung for carbon monoxide, single-breath; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; ICS=inhaled corticosteroid; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; LTOT=long-term oxygen therapy; mMRC=modified Medical Research Council dyspnea scale; SD=standard deviation; VNI=noninvasive ventilation
Citation: Ferraz BD, Cardoso MF, Rodrigues C, et al. Exploring clinical control of COPD: insights from a Portuguese outpatient population. Chronic Obstr Pulm Dis. 2025; 12(5): 390-398. doi: http://doi.org/10.15326/jcopdf.2025.0628
Introduction
Chronic obstructive pulmonary disease (COPD) is a persistent and heterogeneous lung disease characterized by symptoms such as cough, dyspnea, sputum production, and exacerbations.1 The management of COPD poses significant challenges due to the fluctuating nature of its symptoms over time.2,3
Although disease control has been clearly defined in asthma, encompassing the absence of activity limitations, minimal or no symptoms (daytime or nocturnal), minimal or no use of rescue medication, absence of exacerbations, and normal pulmonary function,4,5 this concept has proven more elusive in COPD.6 Indeed, achieving control in COPD patients is often considered unattainable.7
In 2014, Soler-Cataluña et al introduced the concept of COPD control, linked to 2 critical therapeutic dimensions: stability and impact.7 Stability is a longitudinal concept, referring to the absence of significant clinical changes or exacerbations over time. Impact, on the other hand, is a static cross-sectional concept that evaluates the immediate effects of COPD on the patient's daily life at a specific moment.3,7 This framework was subsequently refined and culminated in 2021 with the proposal of comprehensive criteria for assessing COPD control levels, as outlined in Spanish guidelines.8
These updated criteria incorporate exacerbation history, core symptoms, and spirometry results as predictors of short-term exacerbation risk. Stability in this context is measured by the absence of exacerbations in the preceding 3 months and the patient’s subjective perception of their condition. Impact is evaluated based on sputum color, frequency of rescue medication use, activity levels in the past week, dyspnea severity (using the modified Medical Research Council [mMRC] scale), and the degree of airway obstruction quantified by forced expiratory volume in 1 second (FEV1) (Figure 1).9
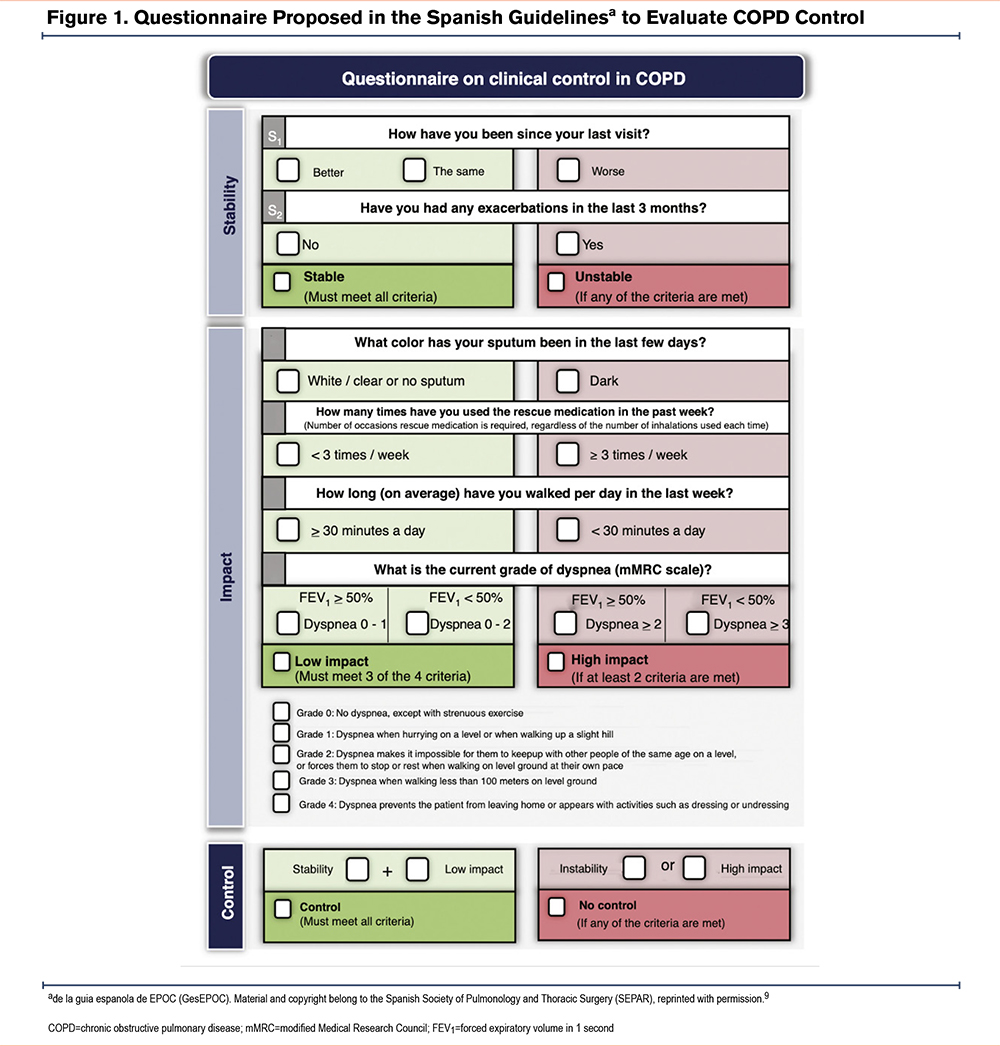
By combining these dimensions, clinicians can assess whether the disease remains stable and whether its impact is low or high; overall disease control is considered achieved when a patient meets the criteria for both stability and low impact.
This control tool has been validated in large COPD cohorts, providing valuable insights into disease management10-12; and it has also shown to be highly informative in tracking changes in the status of COPD patients, demonstrating greater sensitivity than the Global initiative for chronic Obstructive Lung Disease (GOLD)13 A-D classification or clinical phenotypes in predicting changes in clinical stages.14 Besides that, it has also been described that controlled patients have lower symptom load and a better quality of life than uncontrolled patients,11 which reinforces the importance of seeking this stability in COPD patients.
Despite these promising findings, the applicability of COPD control criteria to the Portuguese population remains largely unexamined. Therefore, this study aims to apply the control criteria in a cohort of Portuguese COPD outpatients and to compare exacerbation rates over time between patients classified as controlled versus uncontrolled. Such insights could help optimize COPD management strategies within Portuguese health care settings.
Methods
Study Design
This is a prospective, observational study with a 1-year follow-up in a cohort of patients diagnosed with COPD.
All the cases were assessed in the COPD outpatient clinic on the initial visit (V0), at 6 months (V1), and at 1 year of follow-up (V2). The study was executed in compliance with the Declaration of Helsinki and received approval from the Committee of Ethics and Clinical Investigation of the Unidade Local deSaúde de Santo António.
Patients
The study included patients >40 years of age diagnosed with COPD, defined by the presence of FEV1 to forced vital capacity after bronchodilatation < 0.7, and persistent respiratory symptoms. The exclusion criteria were as follows: active neoplastic disease, inability to carry out follow-up, patients participating in clinical trials, and all patients presenting with an exacerbation within the previous 2 weeks. The recruitment was consecutive, and all patients attending the outpatient COPD clinic who fulfilled the inclusion criteria were considered for the study. The study was explained to them, and those who accepted to participate provided informed consent and were included.
Exacerbations
The absence of exacerbations was established using patient-reported information combined with a review of medical records. This was characterized by the absence of hospitalizations, emergency visits, and ambulatory exacerbations. At V0, this assessment covered the previous 3 months, whereas at V1 and V2, assessment covered the preceding 6-month period.
Clinical Control Status
Patients were classified as controlled or uncontrolled according to a clinical criterion developed by the Spanish Researcher Group.10,12,15 This classification was performed at baseline, and subsequently at 6-month and 12-month follow-up visits.
A patient was considered controlled if the following 2 subcriteria were met: low clinical impact and clinical stability.
Low clinical impact was considered when patients met at least 3 of the following 4 parameters:
- Dyspnea:
- For patients with FEV1 ≥ 50% (assessed at baseline), a dyspnea grade of ≤ 1 on the mMRC scale.
- For patients with FEV1 < 50%, a dyspnea grade of ≤ 2 on the mMRC scale.
- Use of rescue medication: No more than 3 instances of usage within the preceding week.
- Sputum color: White, clear, or no sputum.
- Physical activity: engagement in walking for more than 30 minutes per day during the preceding week.
Clinical stability was considered if both of the following 2 criteria were met:
- Subjective perception of improvement: patients reported their clinical status as "unchanged" or "improved" relative to the previous medical consultation.
- Exacerbations in the last 3 months at baseline or in the last 6 months for follow-up visits, at 6 or 12 months: None.
Patients were also classified according to changes in clinical control status from baseline to 12-month follow-up:
Always controlled: if classified as controlled at baseline and at the 6-month and 12-month follow-up visits;
Always uncontrolled: if classified as uncontrolled at baseline and at the 6-month and 12-month follow-up visits;
Variably controlled: if classified as controlled at least at 1 of the visits and as uncontrolled at least at 1 of the visits.
Unknown: if the patient didn't meet any of the above conditions.
Statistical Analysis
As a first step, patients were categorized as controlled, uncontrolled, or unknown based on clinical criteria at baseline, as well as at the 6-month and 12-month follow-up visits. Next, the proportions of patients in each category were calculated for every visit. However, at the 6-month and 12-month follow-ups, these proportions were further analyzed separately for each classification group from the previous visit. Lastly, the distribution of patients according to changes in clinical control status over the 12-month period—classified as always controlled, always uncontrolled, variably controlled, or unknown—was described as proportions.
Descriptive statistics were used to describe the demographic and clinical characteristics of those classified as controlled and those classified as uncontrolled based on clinical criteria. Categorical variables were described as proportions and compared using Pearson's Chi-squared test or Fisher's exact test, as appropriate. Quantitative variables were described as mean and standard deviation and compared using the independent samples t-test, after the necessary assumptions were checked graphically for all analyses.
All statistical tests were 2-tailed and a P-value lower than 0.05 was considered to be statistically significant. Statistical analyses of the data were performed with SPSS, version 28.0.
Results
A total of 152 patients were initially recruited for the study. However, 19 patients were excluded as they did not meet the inclusion criteria, resulting in a final study sample of 133 patients. At baseline, the mean age of the study sample was 68.1 years old, 73.7% were male, the mean mMRC score was 2.15, the mean COPD Assessment Test (CAT) score was 13.1, and the mean FEV1 (%) was 46.7%. Patients were classified as either controlled (33.1%) or uncontrolled (66.9%) according to the clinical criteria. Of those classified as controlled at baseline, the majority remained controlled (72.7%), and 33.7% of those classified as uncontrolled at baseline were classified as controlled at the 6-month visit (Table 1).
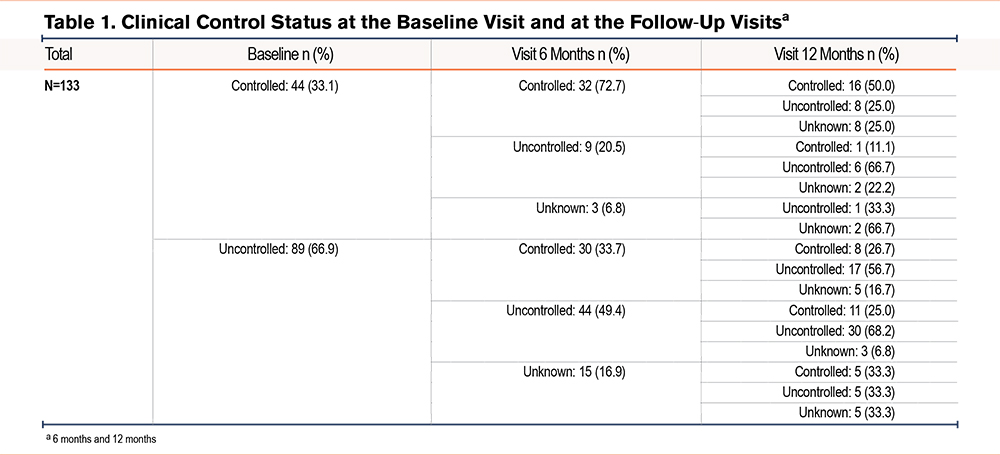
Of the 133 patients, the number classified as controlled increased from 44 (33.1%) at baseline to 62 (46.2%) at the 6-month visit. Overall, the number of patients classified as controlled at the 12-month visit was 42 (31.6% of all 133 patients), but there was an increase in the number of patients who could not be classified. Of those classified as controlled at the 6-month visit, 24/62 (38.7%) remained controlled at the 12-month visit, and of those uncontrolled at the 6-month visit, 12/53 (22.6%) changed to the controlled classification at the 12-month visit. Table 2 summarizes the changes in patients' classification from baseline to the 12-month visit, showing that only 22.6% of patients were always uncontrolled. Although only 12% of the patients were always classified as controlled, a total of 67.7% were controlled at least at 1 of the visits (Table 2).
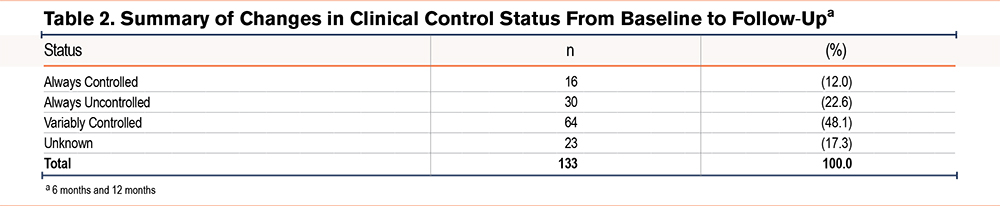
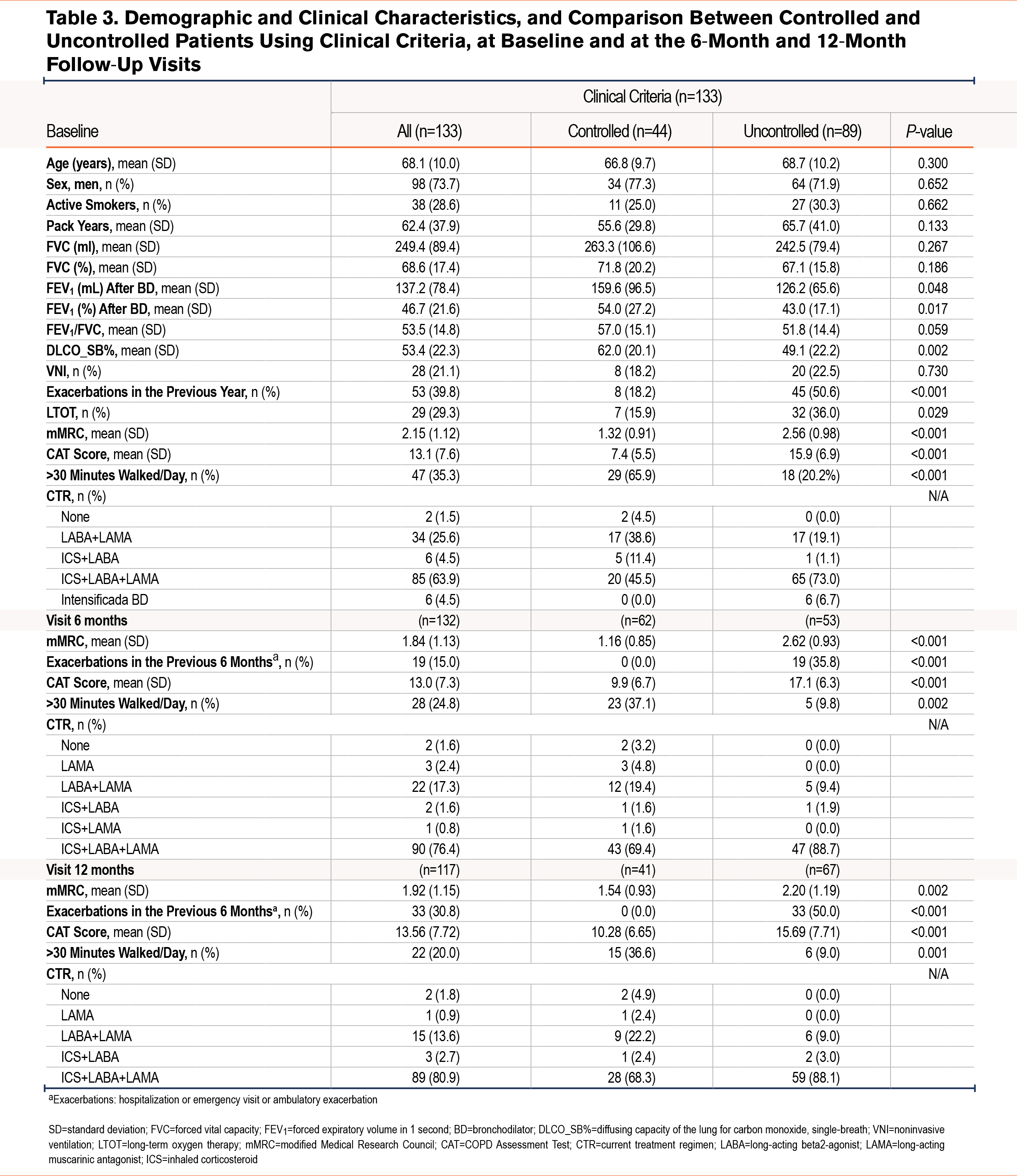
Table 3 shows a comparison of the characteristics of participants classified as controlled and uncontrolled at baseline and at the 6-month and 12-month follow-up visits. At baseline, the 2 groups were similar in terms of age (68.1 ± 10), sex (73.7% male), and tobacco use. The clinically controlled group had significantly higher values for FEV1% and for single-breath diffusing capacity of the lung for carbon monoxide (DLCO_SB%) and a significantly lower occurrence of exacerbations in the previous year and use of long-term oxygen therapy. At all visits, baseline, 6 months, and 12 months, patients classified as controlled had a significantly lower dyspnea grades and CAT scores, and a significantly higher proportion reported walking more than 30 minutes per day.
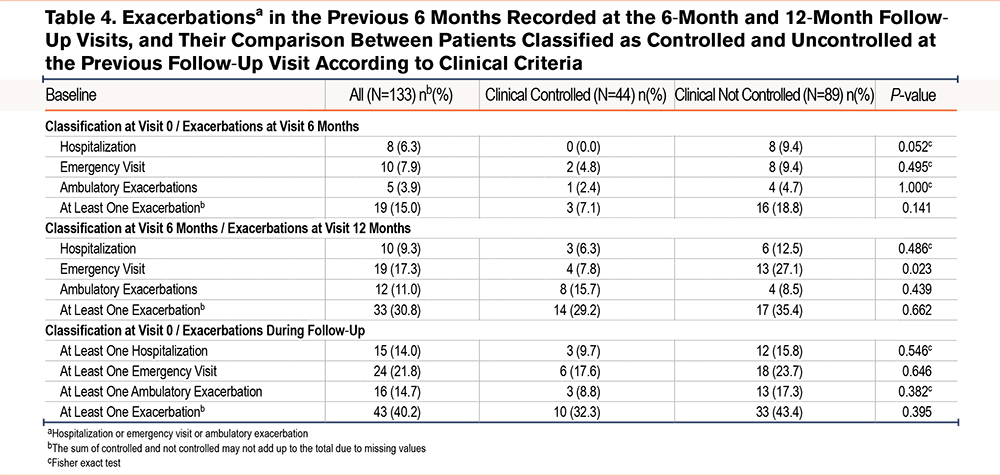
The following outcomes were considered during follow-up: the occurrence of a hospital admission, an emergency visit, an ambulatory exacerbation in the previous 6 months, or having had at least 1 previous exacerbation (Table 4). Overall, only 15% and 30.8% of patients reported having at least 1 exacerbation in the previous 6 months at the 6-month and 12-month visits, respectively. The proportion of exacerbations recorded at the 6-month and 12-month visits was compared between patients classified as controlled and uncontrolled at baseline and the 6-month visit. With only one exception, there was a trend towards an increased proportion of exacerbations in the group of patients classified as uncontrolled at the previous visit, but this increase was only statistically significant for the report of emergency visits at the 12-month follow-up visit (27.1% versus 7.8% in the controlled group, P=0.023). The occurrence of exacerbations during the entire 12-month follow-up period was also compared between patients classified as controlled/uncontrolled at baseline, and although the same trends were observed, none were statistically significant.
Discussion
This study represents the first known validation of COPD control criteria by a research team independent of the original developers and in a Portuguese population. External validation of previously published data is essential for confirming the robustness of any clinical tool, across diverse health care environments and patient populations.
Our findings indicate that only one-third of the patients were classified as controlled at baseline, and merely 12% remained controlled throughout the 12-month follow-up. Notably, half of the patients experienced at least one change in their control status over this period, suggesting that longitudinal fluctuations in disease control are common in this cohort.
We observed a general trend wherein uncontrolled COPD patients experienced more frequent exacerbations than those who were controlled. However, the correlation between exacerbation prediction and clinical control status was weaker than that reported in other studies.10,12,15,16 A possible explanation for this discrepancy lies in our study population, which included patients treated in a dedicated COPD clinic – likely representing individuals with more severe disease. Consequently, these patients typically exhibit poorer lung function, increased symptom burden, and more frequent exacerbations than those managed elsewhere in our center.
This overrepresentation of severe cases may be influenced by several contextual factors. First, our center functions as a regional referral unit for chronic respiratory diseases, receiving a substantial proportion of patients whose conditions could not be adequately managed in primary or general secondary care. This referral pattern introduces a natural bias toward more advanced disease. Second, socioeconomic disparities in our catchment area may contribute to diagnostic delays and reduced access to early treatment interventions, such as smoking cessation programs, inhaled therapies, or pulmonary rehabilitation. Finally, issues related to treatment adherence, either due to limited health literacy or fragmented follow-up in earlier stages, might have contributed to more frequent exacerbations and faster decline. These contextual elements are essential to consider when interpreting the apparent limited predictive value of control criteria in this particular population.
Our results align with a previous study,17 which found that clinical control did not significantly differentiate exacerbation rates among patients with severe COPD (defined as a Body mass index-airflow Obstruction-Dyspnea-Exercise capacity plus exacerbations score > 4). Nonetheless, among patients with mild-to-moderate COPD, that study demonstrated lower exacerbation rates and a longer time to first exacerbation in those who were controlled compared to their uncontrolled counterparts.17 In our study, the mean postbronchodilator FEV1% was 46.7%, which is lower than the values reported in earlier studies.10,15,17 In addition, 29% of the patients in our cohort were under long-term oxygen therapy, compared with only 6.5% and 6.7% in other studies.15,18 These findings underscore the likelihood that our cohort had more severe disease overall; thus, incorporating less severe cases might have enhanced the predictive ability of the criteria.
Another distinguishing feature of our protocol was the use of a single postbronchodilator (FEV1%) measurement (from V0) for both the 6-month (V1) and 12-month (V2) assessments, diverging from the methodology of prior studies.10,15,17 In our clinical setting, repeating spirometry at every visit is not always feasible. Nevertheless, using baseline values might mirror real-world practice conditions and thus, provide additional insights into the applicability and robustness of these criteria.
Assessing disease progression in COPD presents a challenge due to its inherent pathophysiology. Typically, the diagnosis is made at an advanced stage of life when symptoms are already established, and there is no biomarker available to evaluate the disease’s level of activity. Pulmonary function is considered a good method for assessing disease progression, but it also naturally declines over time, making it difficult to distinguish between the effects of COPD and those of aging. Therefore, the concept of remission seems unrealistic in COPD, while the concept of stability, based on clinical and functional criteria, appears to be more appropriate.6
Despite the weaker association with exacerbations in our population, the proposed criteria remain clinically useful. Previous work has shown that patients classified as “controlled” experience fewer symptoms and report a better quality of life.11 Furthermore, it is well established that daily physical activity can reduce the number of exacerbations19,20 and hospitalizations21,22 contributing to the overall control of COPD. Our findings align with this evidence, showing that patients who maintain regular physical activity of at least 30 minutes per day are at lower risk of exacerbation, reinforcing the benefits of regular physical activity. In addition, we observed that uncontrolled patients had a higher number of emergency visits at 6 months compared to controlled patients (Table 4, p=0.023), further supporting the clinical value of the control classification in identifying patients at higher risk for acute care utilization. It is important to note that only COPD-related emergency visits were considered in this analysis. Over the 12-month follow-up period, a total of 29 emergency department visits were recorded: 6 due to disease progression and 23 related to infectious exacerbations. Among the latter, 9 were attributed to bacterial infections (with pathogen identification), 6 to viral infections, and 8 to clinical presentations consistent with respiratory infection—such as increased bronchial secretions and elevated inflammatory markers—but without microbiological confirmation. These data provide further insight into the clinical context of acute events and reflect the burden of both infectious triggers and disease instability in this more severe COPD cohort.
Our data support the ease of application of these criteria in a real-world setting; however, it also highlights the need for further investigations in broader COPD populations to more definitively determine the predictive power and clinical utility of this approach.
Recognizing the concept of disease control in COPD is crucial not only for physicians6 but also for patients,23 as it offers a goal-oriented perspective for disease management. While other studies have validated these criteria in different COPD populations, our findings suggest that additional research focusing on the Portuguese context is warranted, ideally encompassing a wider range of disease severities and multiple clinical settings. Such efforts could more comprehensively illuminate the relationship between “controlled” status and critical outcomes, including exacerbation rates, quality of life, and symptom burden.
A key limitation of our study is the inclusion of a single-center, specialized COPD clinic cohort, which may limit the generalizability of the results to milder cases. Additionally, the lack of repeated postbronchodilator spirometry at each follow-up visit might have influenced the precision of our control assessments. Another important limitation was the small sample size, which restricts the robustness of the findings. Future multicenter studies with larger sample sizes and, preferably, repeated measurements of lung function, are needed to confirm these results and to explore whether the concept of control can inform personalized management strategies that improve long-term patient outcomes. Larger samples would also allow for multivariable analyses of the association between exacerbations and control status, adjusting for potential confounders.
In conclusion, our study provides an independent evaluation of the COPD control criteria in a Portuguese setting, highlighting both their utility and limitations in a more severe disease cohort. Ongoing research in larger, more heterogeneous populations will be necessary to refine the role of these criteria in routine clinical practice and to fully realize their potential in guiding COPD management.
Acknowledgments
Author contributions: BDF and MS worked on the idea and designed the project. BDF, CR, JG, FSC, and CL collected the data. BDF wrote the introduction and discussion. MFC worked on the data analysis and wrote the methods and results and prepared the tables. MS worked in the selection of suitable patients. All the authors reviewed and approved the manuscript.
Declarations of Interest
MS reports consulting fees and honoraria for lectures from Bial, GSK, CSL Behring, and Grifols; support for attending events from Gasoxmed, CSL Behring, Grifols, VitalAire, Bial, and Menarini; and participation on a data safety monitoring board for Sanofi, GSK, and Bial.
BDF, MFC, CR, FC, CL, and JG have no conflicts of interest to declare.