Running Head: Bronchodilator Response in COPD
Funding Support: STR received funding from National Heart, Lung, and Blood Institute T32 grant 5T32HL116271 and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. VNL receives funding from the Cystic Fibrosis Foundation (00003829, 0000075356) and NIH-NHLBI-R01 (HL162171, HL094622).
Date of Acceptance: July 21, 2025 | Published Online: August 1, 2025
Abbreviations: 6MWD=6-minute walk distance; ATS=American Thoracic Society; BDR=bronchodilator response; BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; ERS=European Respiratory Society; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; GLI=Global Lung Function Initiative; GOLD=Global initiative for chronic Obstructive Lung Disease; IQR=interquartile range; SD=standard deviation; SGRQ-C=St George’s Respiratory Questionnaire: COPD; SPIROMICS=SubPopulation and InteRmediate Outcome Measures In COPD Study
Citation: Russell ST, Lama VN, Kempker JA. Bronchodilator response in COPD: definitions, reference equations, and race. Chronic Obstr Pulm Dis. 2025; 12(5): 450-454. doi: http://doi.org/10.15326/jcopdf.2025.0611
Introduction
Bronchodilator response (BDR) is a portion of spirometry which assesses changes in pre- to postbronchodilator forced expiratory volume in 1 second (FEV1) and/or forced vital capacity (FVC). This test is frequently obtained in patients with respiratory complaints and is often used to help differentiate chronic obstructive pulmonary disease (COPD), asthma, or COPD-asthma overlap syndrome. BDR is frequently positive in patients with COPD and its definition has changed. The 2021 American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines now define a positive BDR as a >10% change in FEV1 and/or FVC relative to an individual’s predicted value.1 This predicted value depends on the reference equation applied. Current ATS/ERS guidelines recommend a race neutral reference equation, the Global Lung Function Initiative (GLI)-Global.2 Although studies have compared BDR definitions in patients with COPD, none have used GLI-Global as the reference equation. In a cohort of participants with COPD, we compared the frequency of a positive BDR between the 2005 and 2021 definitions, using both GLI race-specific and race-neutral reference equations, stratified by race.
Methods
We performed a cross-sectional secondary analysis of the SubPopulation and InteRmediate Outcome Measures In COPD Study (SPIROMICS). SPIROMICS was a multicenter cohort study of participants with or at risk for COPD from 2010–2015. Participants were aged 40–80, and could have a previous history of asthma but not a primary diagnosis of asthma. Participants completed pre- and postbronchodilator spirometry, questionnaires, and a 6-minute walk test at the baseline visit.
We included participants in SPIROMICS with COPD at the baseline visit, defined as FEV1/FVC < 0.7. We excluded races other than White and Black due to a low sample size. For each patient, we determined BDR based on the 2005 (BDR-05) or two 2021 definitions (BDR-21). The 2005 definition for a positive BDR is an increase in postbronchodilator FEV1 and/or FVC greater than or equal to 12% and 200mL compared with prebronchodilator raw values.3 The 2021 ATS/ERS guidelines define a positive BDR as a >10% change in FEV1 and/or FVC relative to an individual’s predicted value.1 The two 2021 BDR definitions used GLI-race specific and GLI-Global reference equations, respectively. Participant race was self-reported. The predicted values were obtained from the GLI calculator.4 SPIROMICS data was obtained via the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center.
Our primary outcome was frequency of positive BDR according to each definition, stratified by race. Subsequent analyses used BDR-21 with GLI-Global reference ranges, based on current ATS recommendations. We tested the overall agreement between definitions using the Kappa statistic. We subsequently defined 4 strata: BDR-05 + / BDR-21 +, BDR-05 - / BDR-21 -, BDR-05 +/ BDR-21 -, BDR-05 - / BDR-21 +. Across these strata we compared spirometry values, St George’s Respiratory Questionnaire-COPD (SGRQ-C), and 6-minute walk distance (6MWD). Continuous variables were compared using Anova or T-test for normally distributed variables and Wilcoxon rank sum test or Kruskal-Wallis for nonparametric variables. Categorical variables were compared with Chi-squared tests. Statistical Analysis was completed using SAS 9.4 (SAS Inc., Cary, North Carolina). Institutional review board approval was granted by Emory University.
Results
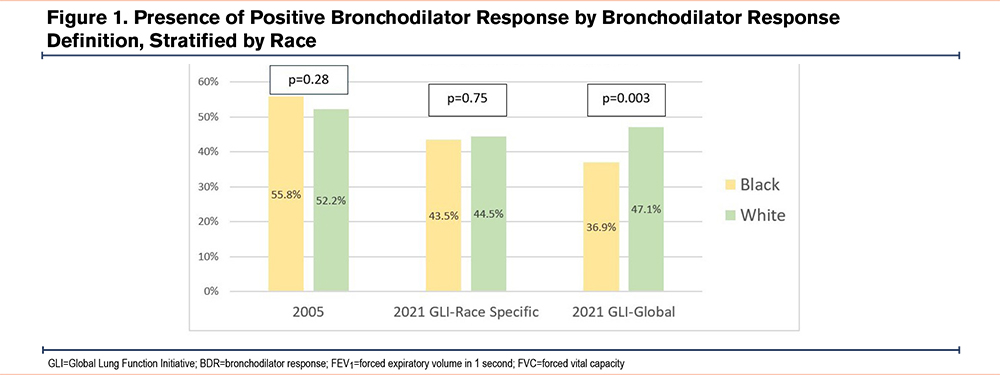
There were 1688 participants analyzed. A total of 1428 (84.6%) were White and 260 (15.4%) were Black. A total of 745 (52.2%) White participants had a positive BDR-05 and 672 (47.1%) had a positive BDR-21. A total of 145 (55.8%) Black participants had a positive BDR-05 and 96 (36.9%) had a positive BDR-21. White and Black participants had different frequencies of a positive BDR using BDR-21 with GLI-Global reference equations (p=0.003), but not the other definitions (Figure 1). The Kappa statistic for agreement between BDR-05 and BDR-21 was 0.8 (95% confidence interval [CI]: 0.77–0.83) for White participants and 0.61 (95% CI: 0.53–0.71) for Black participants.
Black participants with BDR-05 + / BDR-21 - had a lower percentage predicted FEV1 (37.82, interquartile range [IQR]: 32.17; p=0.002) than either concordant positive BDR (50.90, IQR: 28.11) or concordant negative BDR (57.36, IQR: 32.24). A similar pattern was noted for FVC (Table 1). A total of 56.9% of Black participants with BDR-05 + / BDR-21 – had Global initiative for chronic Obstructive Lung Disease (GOLD)5 stage 3 or 4 severity, compared with 35.8% of those with concordant positive and 33.3% with concordant negative BDR. There were no differences in 6MWD (p=0.48) or SGRQ-C (p=0.59) (Table 1).
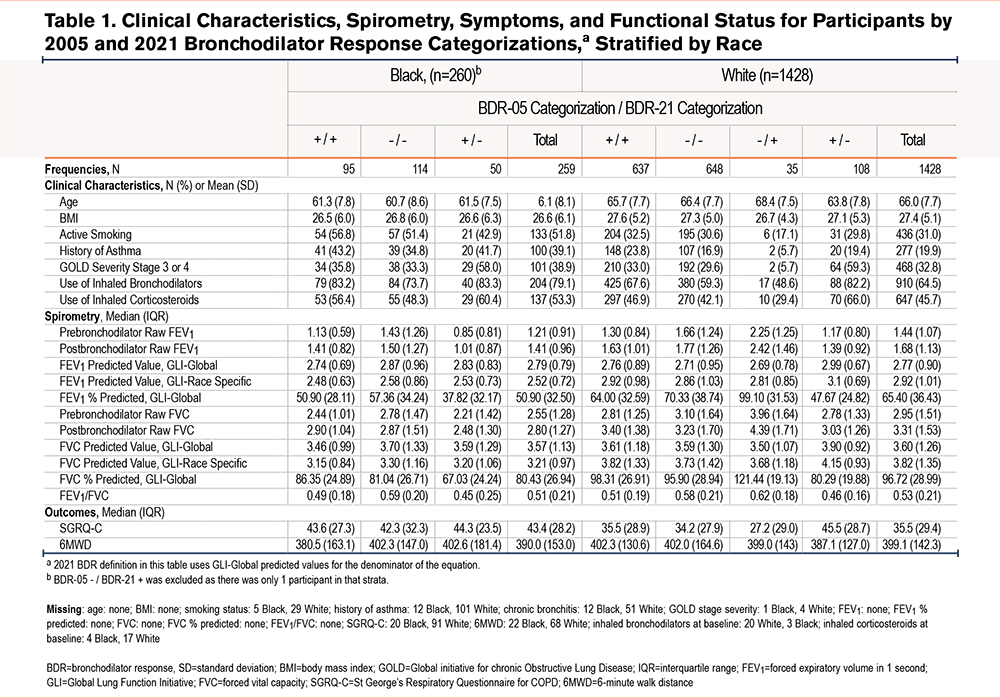
White participants with BDR-05 + / BDR-21 – had a lower percentage predicted FEV1 (47.67, IQR 24.82; p<0.001) than concordant positive BDR (64.00, IQR: 32.59), concordant negative BDR (FEV1 70.33, IQR 38.74), or BDR-05 - / BDR-21 + (99.10, IQR: 31.53). A total of 59.3% of White participants with BDR-05 + / BDR-21 - had COPD severity grades 3 or 4, compared with 33.0% with concordant positive BDR, 29.6% concordant negative BDR, and 5.7% with BDR-05 - / BDR-21 +. White participants with BDR-05 + / BDR-21 – had a higher SGRQ-C (45.5, IQR: 28.7; p<0.001) than the other 3 BDR strata. There were no differences in 6MWD, p=0.86 (Table 1).
Discussion
In this study, fewer Black than White patients with COPD had a positive BDR-21 using race neutral spirometry reference equations. This difference is not present with BDR-21 using race specific reference ranges or with BDR-05. Across both races, participants with a positive BDR-05 but negative BDR-21 had lower spirometry values and more severe GOLD stages.
The strength of this study is the well phenotyped, large size of the SPIROMICS cohort. The primary limitation is that our analysis was limited to White and Black participants, due to low numbers in other race groups. Our findings may not be applicable to other racial/ethnic groups.
Multiple studies have compared BDR definitions in COPD cohorts.6-10 Bhatt et al10 found an increase in BDR-21 compared with BDR-05, from 32.5% to 44.6%. This study excluded participants with self-reported asthma and used race-specific GLI reference ranges for BDR-21. Beasley et al7showed a decrease in positive BDR from 24.7% by BDR-21 to 18.0% by BDR-05. The directionality of BDR changes in our study aligns with Beasley, however our overall number of BDR positive participants is higher. This difference is possibly due to the inclusion of patients with a self-reported history of asthma.
There has been significant debate in the pulmonary community regarding the impact of race-specific versus race-neutral reference ranges, and this study adds to that literature.2,11,12 We tested the BDR definitions on the same participants, who, therefore, acted as their own controls. Using race-neutral GLI reference ranges, compared with race-specific GLI reference ranges, Black participants had a decrease in the frequency of positive BDR of 6.6% (43.5% to 36.9%) while White participants had an increase of 2.6% (44.5% to 47.1%). This directionality is due to the fact that the predicted spirometry values for FEV1 and FVC, which forms the denominator of the BDR equation, is relatively higher for Black individuals and lower for White individuals using GLI-Global compared with GLI-race specific equations (Table 1).12 The BDR changes therefore reflect definitional rather than physiologic or biologic differences and, as there is no gold standard, an optimal BDR definition should correlate with patients’ related outcomes. While in line with current ATS guidelines, applying the 2021 definition for BDR with GLI-Global reference ranges leads to higher identified BDR in White participants compared with Black participants and may introduce disparities in the diagnosis and care of patients with respiratory complaints and airway-based diseases if BDR is used as a criteria for concurrent asthma diagnosis, specific inhaler therapies, or clinical trial inclusion criteria in patients with COPD.1,2
Acknowledgements
Author contributions: STR and JAK were responsible for conceptualization, study design, and data analysis. STR, JAK, and VNL provided data interpretation and manuscript editing. STR was responsible for data acquisition and drafting the manuscript. All authors reviewed and approved the final manuscript submitted for publication.
Data sharing statement: Deidentified SPIROMICS data is available via request through the National Heart, Lung, and Blood Institutes Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) or direct request from SPIROMICS study PI’s. We obtained the data through the BioLINCC request but in accordance with that agreement are not able to share data directly, however SPIROMICS data is readily available to all through the above mechanisms.
Declaration of Interest
STR, VNL, and JAK declare that there are no conflicts of interest in relation to this manuscript.