Running Head: Low Lung Function Increases Mortality Risk
Funding Support: None
Date of Acceptance: July 25, 2025 | Published Online: August 1, 2025
Abbreviations: BMI=body mass index; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; FEF25%–75%=forced expiratory flow between 25% and 75% of vital capacity of the predicted normal value in liters/second; FEV1=forced expiratory volume in 1 second; FEV1%pred=FEV1 percentage predicted; FVC=forced vital capacity; GOLD=Global initiative for chronic Obstructive Lung Disease; HLF=high lung function; HRQoL=health-related quality of life; IRB=institutional review board; LLF=low lung function; LSC=Lovelace Smokers’ Cohort; MCID=minimally important clinical difference; MLF=moderate lung function; OR=odds ratio; SD=standard deviation; SGRQ=St George’s Respiratory Questionnaire
Citation: Tesfaigzi Y, Brown MN, Liu C, et al. Low lung function in middle-aged smokers impacts health status, morbidities, and mortality: an observational analysis of the Lovelace smokers cohort. Chronic Obstr Pulm Dis. 2025; 12(5): 357-367. doi: http://doi.org/10.15326/jcopdf.2025.0605
Introduction
The presence and severity of lung disease is often determined by measuring lung function. First systematically studied by Hutchinson in 1846 using the spirometer and referred to as vital capacity,1 the modified forced maneuver of vital capacity (forced vital capacity [FVC]) relates well to future risk of death.2,3 From spirometry, the ratio of forced expiratory volume in 1 second (FEV1) to FVC (FEV1/FVC) is used to confirm the presence of airway obstruction,4 which can be caused by diseases such as asthma or chronic obstructive pulmonary disease (COPD).5
Lung function measurements (e.g., FEV1 and FVC obtained from spirometry) are not typically performed in younger individuals suspected to be at risk for obstruction, but several studies have shown that these measures may be predictive of health outcomes later in life.3,6-9 In a study of 3 separate patient cohorts, Agusti et al reported patients aged 25–40 years with a FEV1 percentage predicted (FEV1%pred) <80% had higher prevalence of respiratory, cardiovascular, and metabolomic abnormalities in early adulthood, higher and earlier incidence of comorbidities during follow-ups, and greater all-cause mortality risk compared with patients with an FEV1%pred >80% at 25–40 years of age.6 Importantly, the relationship between low FEV1%pred and all-cause mortality risk was independent of, but additive to, cumulative smoking exposure in the Framingham Offspring Cohort.6 However, patient-related measures of health-related quality of life (HRQoL) were not reported in that study. In 2022, Labaki et al analyzed the association between cause of death and the degree of lung function impairment in smokers aged approximately 60 years (± 9 years) enrolled in the COPD Genetic Epidemiology (COPDGene®) study.10 Over a 17-year median observation period, certain comorbidities, particularly cardiovascular diseases and lung cancer, were reported to be more frequent in smokers with mild spirometry abnormalities than in smokers with normal spirometry.10 However, information regarding the individual’s perception of their health status is limited. Additionally, longitudinal incidence of new comorbidities has not been reported, and there is a lack of longitudinal studies examining relationships between lung function and tobacco smoking at younger ages on long-term health outcomes in those at-risk for COPD.
We hypothesized that lung function, as measured by FEV1 and FVC, may be associated with pulmonary and nonpulmonary morbidities and impaired HRQoL, and could be predictive of long-term mortality risk in middle-aged smokers. As such, we leveraged a subpopulation of participants aged 40–60 years (the youngest age group of smokers commonly having spirometry results) from the Lovelace Smokers’ Cohort (LSC), a unique longitudinal cohort of current or former smokers from a broad community setting. In this cohort, we evaluated long-term outcomes in middle-aged current or former smokers with low versus high lung function (HLF), as indexed by the FEV1%pred.
Methods
Population
This study included participants from the previously described LSC.7,11,12 In brief, the LSC recruited current or ever smokers with a smoking history of ≥10 pack years aged 40–75 years from a broad community setting in Albuquerque, New Mexico (and surrounding communities)7 from 2001–2011. The LSC began recruiting female smokers in 2001, expanding to male smokers13 in 2004. Female ever smokers were favored in the LSC to study female susceptibility to the adverse effects of smoking, given that they are underrepresented in most COPD studies.14 Participants were generally recruited through newspaper or television advertisements and were paid for their participation.12 All participants signed an institutional review board (IRB)-approved informed consent form prior to any study-related procedures. The IRB of record for study conduct was Advarra IRB. Assessments and measures regularly conducted/obtained from LSC participants included HRQoL assessments (the 36-Item Short Form Health Survey), exacerbation history, medication history, modified Medical Research Council dyspnea score, comorbidities, and smoking status. Spirometry was performed by trained technicians following European Respiratory Society/American Thoracic Society standards.15 The St George’s Respiratory Questionnaire (SGRQ) was used to score HRQoL.16
These analyses included male and female participants from the LSC aged 40–60 years at baseline, without airway obstruction (obstruction was defined as FEV1/FVC <0.7) and with at least 2 follow-up visits. The Global initiative for chronic Obstructive Lung Disease (GOLD) 2024 report classifies younger adults with COPD as those aged <50 years.17 For this analysis, the age range of 40–60 years was selected to account for the 17-year follow-up period and to allow for a sufficient population size for analysis. Participants were assigned to the overall cohort and subsequently grouped into lung function tertiles based on baseline FEV1%pred (low lung function tertile [LLF], baseline FEV1%pred <88%; moderate lung function, baseline FEV1%pred ranging from 88%–99%; HLF, baseline FEV1%pred >99%). As this study aimed to compare participants with low versus HLF, only the LLF and HLF tertiles were included in these analyses. In addition, a subset of participants from the LLF and HLF tertiles were recruited for a single clinical visit completed during 2021 and 2022, and comprised the 17-year long-term follow-up cohort.
Study Variables and Outcomes
Baseline demographics, anthropometrics (including body mass index [BMI]), and clinical characteristics (including smoking history, lung function measures [based on FEV1, FEV1%pred, and FVC], HRQoL, and comorbidities) were assessed in the LLF and HLF tertiles for the overall cohort and 17-year long-term follow-up cohort. Chronic bronchitis was defined by self-reported cough and phlegm for at least 3 months over 2 years. All-cause mortality was determined by contacting the household and by searches of the National Death Index.
Data Analysis
Baseline characteristics in the LLF and HLF tertiles are reported using descriptive statistics. Between-tertile differences in comorbidities at baseline and mortality are reported using odds ratios (ORs) with 95% confidence intervals (CIs), as previously published for this cohort.18 We used the Cox model to compare survival between the LLF and HLF groups after adjusting for age, sex, BMI, pack years, current smoking, and the comorbidities of wheeze, cardiovascular disease (CVD), respiratory diseases, and diabetes. Logistic regression analyzed associations between baseline lung function and self-reported baseline comorbidities, both of which were adjusted for age, sex, BMI, race, pack years of smoking, and smoking status. Differences in comorbidities, lung function, and health status over time between the cohorts are reported using descriptive statistics, with between-group comparisons examined using Chi-squared tests. P-values are nominal and not adjusted for type I error.
Results
Participant Disposition
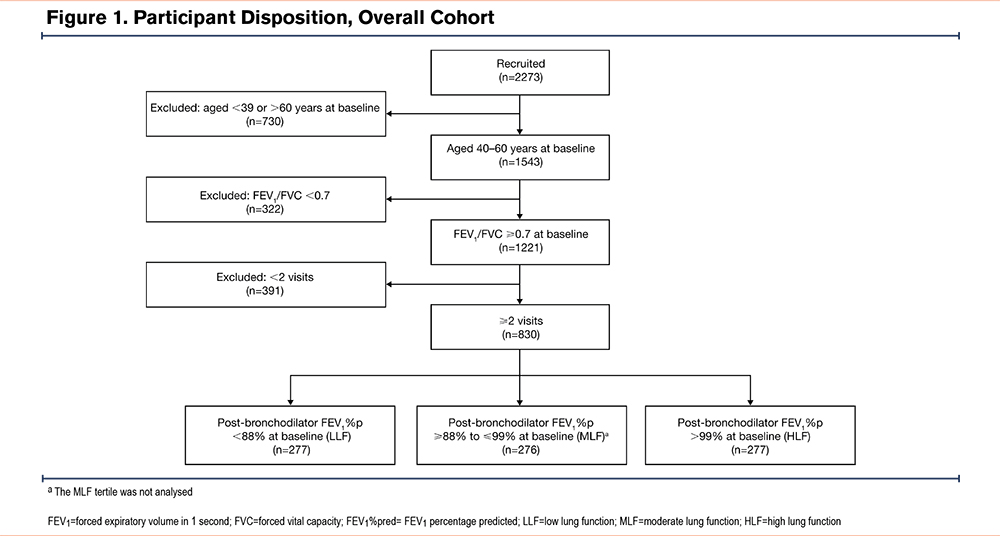
Figure 1 reports participant disposition. In total, from 2273 LSC participants, 830 met the eligibility criteria and were included in the analysis. Of these, 277 participants comprised each of the tertiles. Eighty-seven participants (LLF, n=26; HLF, n=61) were recruited to a follow-up clinical visit in 2021–2022 and were included in the 17-year long-term follow-up cohort.
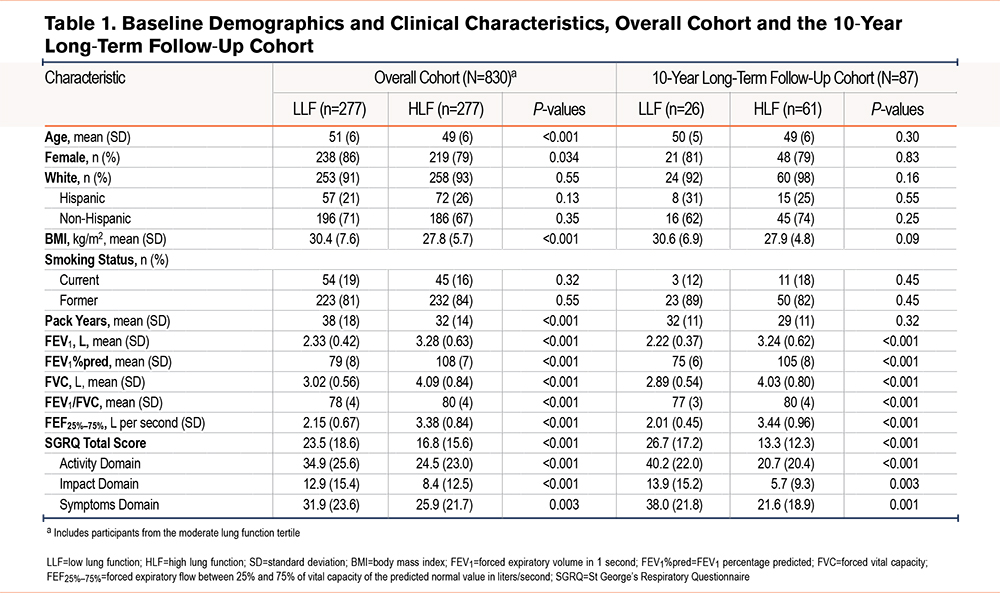
Participant Demographics and Clinical Characteristics
Table 1 reports baseline demographics and clinical characteristics for the overall and 17-year long-term follow-up cohorts. Except for lung function and BMI, the baseline demographic and clinical characteristics show that the LLF group was more likely to be older, female, have more pack years, and have a higher BMI than the HLF group (Table 1). Mean age at baseline was 51 and 49 years, 86% and 79% of participants were female, and 71% and 67% were non-Hispanic White in the LLF and HLF tertiles, respectively. Mean BMI was 30kg/m2 in the LLF tertile and 28kg/m2 in the HLF tertile. As expected, mean lung function measures (as determined by FEV1%pred) were lower in the LLF tertile versus the HLF tertile: FEV1 (2.33 L versus 3.28 L), FEV1%pred (79% versus 108%), and FVC (3.02L versus 4.09L).
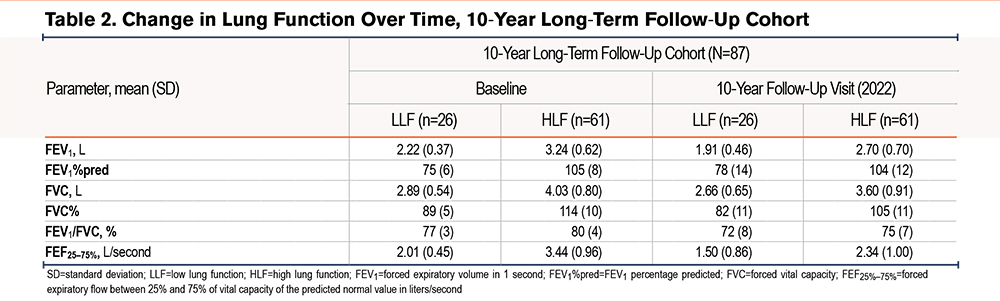
In the long-term follow-up cohort, baseline demographics and clinical characteristics were generally similar to the overall cohort (Table 1). In the LLF and HLF tertiles, mean baseline age was 50 and 49 years, 81% and 79% of participants were female, and 62% and 74% of participants were non-Hispanic White, respectively. Mean BMI was approximately 11% higher in the LLF tertile (31kg/m2) versus the HLF tertile (28kg/m2). Lung function measures were consistently lower in the LLF versus HLF tertile, with mean FEV1, FEV1%pred, and FVC being 2.22 L and 3.24 L, 75% and 105%, and 2.89 L and 4.03 L, respectively.
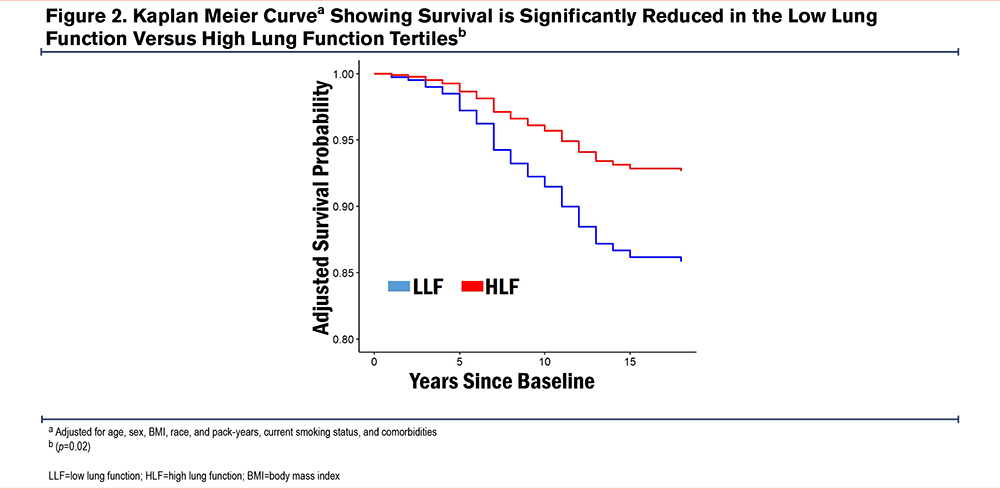
Risk of All-Cause Mortality
In the overall cohort, LLF at baseline was associated with significantly higher all-cause mortality rate versus HLF (16.3% versus 6.5%). The OR (95% CI) for LLF relative to HLF was 2.29 (1.22, 4.46); p=0.01. The Kaplan Meier survival curve shown in Figure 2 confirms this difference over time after adjusting for age, sex, BMI, race, pack years, current smoking status, and comorbidities (p≤0.02).
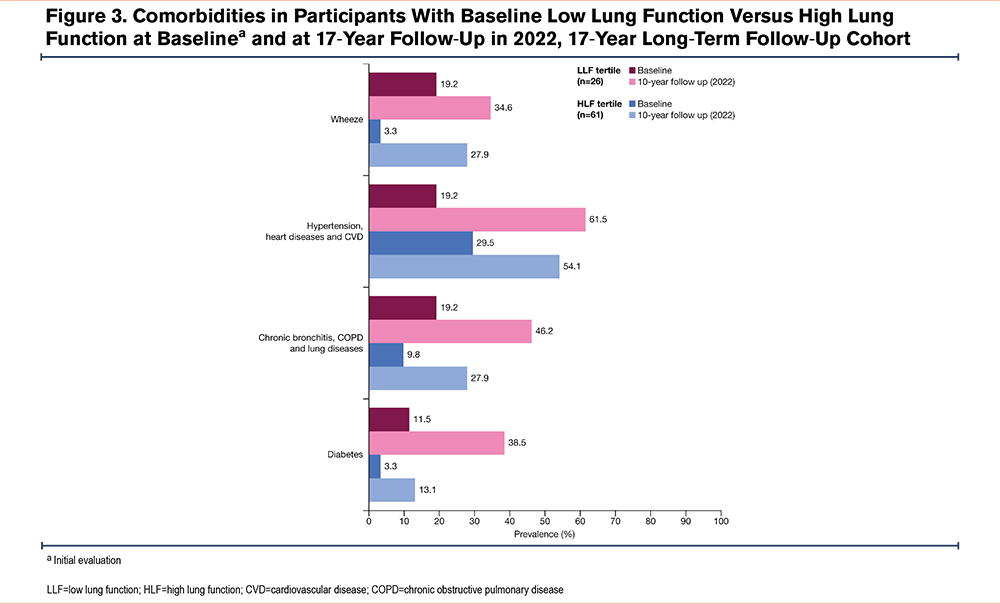
Self-Reported Comorbidities
Baseline and Long-Term Follow-Up Cohort
In the overall cohort, at baseline, the proportion of participants reporting baseline CVD was better in the LLF compared with the HLF group, while all other comorbidities were worse in the LLF versus HLF tertile, although these differences failed to reach statistical significance (Figure 3). At baseline, the LLF relative to HLF showed no differences for the self-reported diabetes (OR [95% CI]: 6.88 [0.73, 153.2]; p=0.10) and calculated chronic bronchitis and COPD (OR [95% CI]: 1.65 [0.30, 8.85]; p=0.55), CVD (OR [95% CI]: 0.63 [0.17, 2.04]), and wheeze CVD (OR [95% CI]: 6.51 [0.96, 60.02]). At the 17-year follow-up, the LLF relative to HLF, lower proportions of study participants had self-reported diabetes (OR [95% CI]: 6.73 [1.05, 51.56]; p=0.05) and calculated chronic bronchitis and COPD (OR [95% CI]: 7.74 [1.51, 56.02]; p=0.02), CVD (OR [95% CI]: 1.58 [0.36, 7.51]), and wheeze CVD (OR [95% CI]: 2.78 [0,52, 16.34]).
After the 17 years, most self-reported comorbidities were more prevalent in the LLF tertile versus the HLF tertile. The greatest numerical differences at follow-up were observed for chronic bronchitis, COPD, and lung disease (LLF, 46.2%; HLF, 27.9%; difference, 18.3%) and diabetes (LLF, 38.5%; HLF, 13.1%; difference, 25.4%), as shown in Figure 3.
St George’s Respiratory Questionnaire Score
Baseline
In the overall cohort, the LLF tertile had worse baseline health status, as indicated by higher SGRQ total (p<0.001) and domain scores (all p≤0.003) versus the HLF tertile (Table 1). In the 17-year follow-up cohort, mean SGRQ total scores were approximately 24 in the LLF tertile and 17 in the HLF tertile (Table 1).
Long-Term Follow-Up Cohort
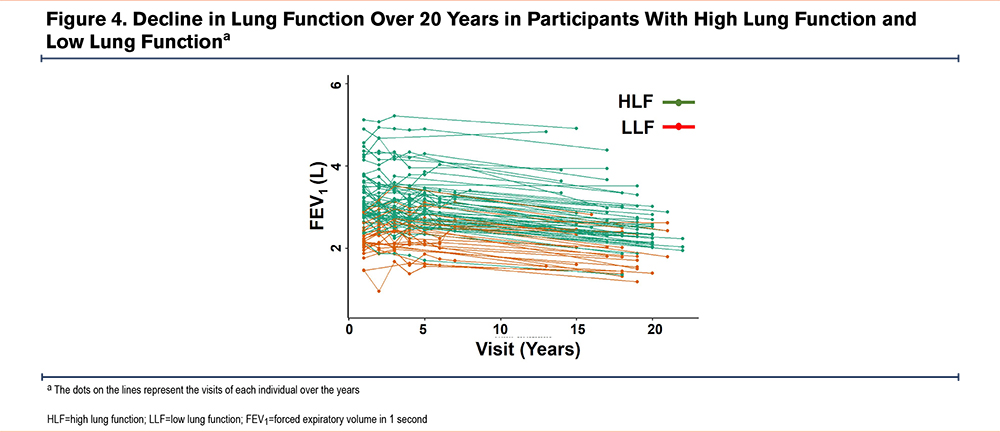
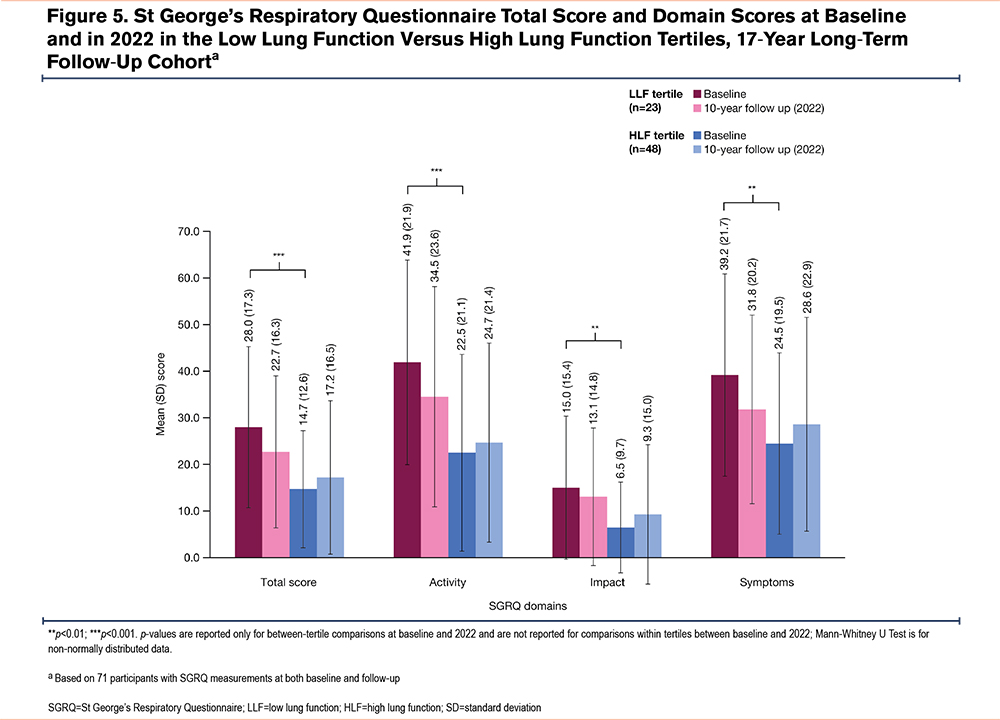
In the long-term follow-up cohort, SGRQ score changes were analyzed in 71 participants with SGRQ data at baseline and follow-up (LLF, n=23 and HLF, n=48). As in the overall cohort, baseline SGRQ total (p<0.001) and domain scores (all p≤0.003) were higher in the long-term follow-up cohort, indicating worse health status with LLF versus HLF (Table 1); mean total SGRQ scores were approximately 27 and 13, respectively in the LLF and HLF tertiles. When assessing SGRQ changes over time, total and domain scores improved from baseline to follow-up in the LLF tertile (mean SGRQ total score decreased from approximately 28 to 23; Figure 4), whereas in the HLF tertile, scores worsened (mean SGRQ total score increased from approximately 15 to 17; Figure 4). In general, mean SGRQ total and domain scores remained numerically higher in the LLF tertile versus the HLF tertile at follow-up (Figure 5).
Lung Function
Long-Term Follow-Up Cohort
Lung function measures, including FEV1, FEV1%pred, and FVC, were lower in the LLF tertile versus the HLF tertile in the 17-year long-term follow-up cohort at baseline and follow-up (Table 2). Mean FVC decreased in the LLF and HLF tertiles, with reductions of 0.23L and 0.43L, respectively. The decline in FEV1 was similar in both groups over the 17 years of observation (Figure 4). In the HLF and LLF groups, 21% and 49% transitioned into FEV1/FVC <70, respectively, over this time period.
Discussion
In this middle-aged cohort (aged 40–60 years), current or ever smokers without baseline lung airflow obstruction, participants with LLF (based on FEV1%pred) were at increased all-cause mortality risk versus those with higher lung function. Participants with LLF also had significantly worse baseline HRQoL (both clinically and statistically) versus those with HLF, with SGRQ total score differences exceeding minimally important clinical differences (MCID)19 by nearly 2-fold in the overall cohort and more than 3-fold in the 17-year long-term follow-up cohort. Additionally, participants with LLF had a greater prevalence of multiple clinically important comorbidities versus participants with HLF. Importantly, differences in comorbidity prevalence between participants with LLF versus HLF widened over time. These findings indicate that LLF was a predictor of health outcomes independent of smoking history in this middle-aged cohort of current or ever smokers. We used the Global Lung Function Initiative equations and did not see any difference in outcomes. This is consistent with the findings of the study of longitudinal data from 369,077 participants in the National Health and Nutrition Examination Survey, the UK Biobank, the Multi-Ethnic Study of Atherosclerosis, and the Organ Procurement and Transplantation Network. In that study, race-based and race-neutral equations generated similarly accurate predictions of respiratory outcomes.20
The findings of increased risk of all-cause mortality in middle-aged smokers with LLF versus HLF expand on previously published findings from Agustí et al in a transgenerational cohort study, which reported greater mortality risk in young adults with lower versus higher lung function.6 However, in that study, cigarette smoking exposure and the proportion of current or ever smokers were significantly higher in participants with LLF. Although corrections for tobacco exposure were made by Agustí et al, these differences may have partially explained the difference in mortality between the study populations. This was not the case in the current study, where smoking history was similar between the LLF and HLF tertiles, as all LSC participants were current or ever smokers, and percentages of current smokers were roughly comparable between tertiles. It is worth noting that Ashley et al previously reported FVC was a predictor of survival in participants from the original Framingham Heart Study cohort, but further associations between FVC and comorbidities or HRQoL were not described.3 Together, our study strongly supports the concept that lower spirometry values (specifically lower FEV1%pred) within the normal range in participants without airway obstruction are associated with increased mortality risk, independent of smoking history (Figure 2).
A novel finding of this study is the higher baseline SGRQ scores in participants with LLF versus HLF (Figure 5). The absolute difference of approximately 13 points in the 17-year follow-up cohort is more than 3 times the estimated MCID of 4 points for the SGRQ total score,19 and thus, indicative of worse baseline HRQoL in participants with LLF. Interestingly, SGRQ scores increased from baseline to follow-up in the HLF tertile, but the difference between tertiles remained larger than the MCID for SGRQ total score, even though there were more deaths in the LLF tertile, which likely selected out participants with the highest (i.e., worst) SGRQ scores. While it is known that more severe airflow obstruction is associated with higher SGRQ scores in patients with COPD,21,22 airway obstruction is unlikely the primary factor driving the association between LLF and higher SGRQ observed in this study, as mean FEV1/FVC was only slightly lower in the LLF versus the HLF tertile. The substantial difference of approximately 1L in FVC and FEV1 and the 2.6kg/m2 difference in BMI between the tertiles may have contributed to SGRQ score differences. However, the small difference in BMI is not likely to alter the mortality risk in smokers, even if they have COPD, in which higher BMI can protect against mortality.23,24
A further important finding is the greater prevalence of several important comorbidities in participants with LLF versus HLF, which could explain the increased mortality risk and worse HRQoL in participants with LLF. These results are consistent with greater baseline prevalence and future incidence of respiratory, cardiovascular, or metabolic abnormalities in young adults aged 25–40 years with lower versus normal lung function observed in 2 nonoverlapping young adult cohorts, reported by Agustí et al.6 In that study, a significantly greater proportion of participants with LLF had baseline respiratory or cardiovascular abnormalities relative to participants with normal lung function, with a numerically greater proportion of participants with LLF also having metabolic abnormalities.6 At follow-up, participants with LLF had a greater incidence and earlier occurrence (by approximately a decade) of morbidities relative to participants with normal lung function.6 As in this study, baseline LLF was associated with a greater mortality risk (hazard ratio [95% CI]: 2.3 [1.4, 3.7]) in Augusti et al,6 but no HRQoL measurements were reported. The significant differences in SGRQ scores observed in this study may be partially driven by a difference in the prevalence of baseline comorbidities between participants with HLF and LLF, given that they are unlikely to be explained solely by lung function differences.
Previous studies have suggested COPD represents a disease of accelerated aging, characterized by genomic instability, telomere attrition, premature cellular senescence, and other hallmarks of aging.25,26 One study reported patients with confirmed COPD aged 56–65 years had a similar number of comorbid diseases associated with aging as those without COPD, 15 to 20 years older, irrespective of smoking history.26 For example, the prevalence of atherosclerosis and osteoporosis in patients with COPD aged 56–65 was 1.22% and 9.67%, respectively, versus 0.23% and 5.85% in similarly aged people without COPD and 1.12% and 6.58% in people aged >85 years without COPD.26 Similar results were observed for other comorbidities associated with aging, including chronic renal failure, coronary artery disease, and hearing loss.26 This study suggests LLF in ever smokers without COPD may similarly predict accelerated aging, as participants with baseline LLF had a greater prevalence of chronic diseases than those with HLF, including hypertension, heart or cardiovascular disease, chronic bronchitis, and diabetes.
While it is known smoking is associated with lung function decline, the rate of FEV1 decline in male and female smokers who quit before the age of 30 is indistinguishable from that of healthy never smokers.27 In contrast, smokers who quit after the age of 40 have significantly higher rates of FEV1 decline compared with healthy never smokers and early quitters.27 Therefore, early clinical assessment and intervention with the aim of encouraging smoking cessation at an early age before development of long-term lung function decline or symptoms of COPD is warranted. Indeed, pre-COPD—which identifies patients at any age with respiratory symptoms or other structural/functional pulmonary abnormalities without airflow obstruction28—was raised in the GOLD 2024 report,17 emphasizing the importance of acknowledging the risk of developing COPD in smokers with LLF and other relevant clinical characteristics.
This study has several strengths, including the use of a large population of smokers without airflow obstruction, of which a substantial proportion were female. Additionally, participants were very well phenotyped, helping evaluate pulmonary and extrapulmonary domains, including self-reported morbidities and HRQoL. Importantly, 87 participants provided 17-year follow-up data, thus providing longitudinal data to evaluate the long-term effects of low versus HLF on outcomes. There are also some limitations to consider. First, relative to participants with HLF, notably fewer participants with LLF returned for the 17-year follow-up visit. The lower number of participants with LLF who returned for follow-up may have minimized true differences between LLF and HLF tertiles, as those with LLF had more severe illness. Second, the high proportion of women may limit the applicability of these results to males. However, the similar findings reported in other studies comprising primarily men suggest the results are valid for both sexes.
Conclusions
These results indicate that LLF, relative to HLF, in ever smokers without COPD is associated with increased all-cause mortality risk, clinically significant worse HRQoL, and the development of chronic morbidities indicative of accelerated aging. These data suggest the use of a simple spirometry test to detect lower than normal lung function can help identify at-risk patients in clinical practice, allowing for an earlier emphasis on interventions, such as smoking cessation, which can improve long-term health outcomes in this understudied population. Discussion of pre-COPD in the GOLD 2024 report17 emphasizes the importance of acknowledging the risk of developing COPD in smokers with reduced lung function but without airway obstruction. Indeed, these middle-aged smokers may benefit substantially from intervention, given that smoking cessation significantly improves long-term rates of lung-function decline.
Acknowledgements
Author contributions: MNB, BRC, PD, and YT contributed to the conceptualization of the manuscript. FXB, BRC, CL, KO, MAP, MP, and YT were responsible for the data curation. MNB, BRC, CL, and KO contributed to the formal analysis of the manuscript. MNB, PD, KO, and YT were in charge of the funding acquisition. FXB, BRC, PD, DH, CL, KO, MP, and YT contributed to the investigation. MNB, FXB, BRC, PD, CL, KO, MP, and YT contributed to the methodology. MNB, FXB, BRC, DH, KO, MP, and YT were in charge of the project administration. SAB, FXB, CL, KO, MP, and YT were in charge of the resources. CL was responsible for the software. BRC, PD, DH, KO, MP, and YT were in charge of the supervision. BRC was responsible for the visualization. SAB, FXB, MNB, BRC, PD, DH, VAH, CL, KO, MP, MAP, and YT contributed to the writing, reviewing, and editing of the manuscript. All authors provided final approval of the manuscript submitted for publication.
Other acknowledgements: Medical writing support, under the guidance of the authors, was provided by Daniel Spindlow, MSc, and Rebecca Douglas, PhD, of CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines.
Data sharing statement: Due to privacy regulations with the Lovelace Smokers’ Cohort, the clinical data from this study cannot be disclosed.
Declarations of Interest
YT reports consulting fees for Verra Therapeutics. FXB, KO, and MP are employees of AstraZeneca and hold stock/stock options in the company. VAH (currently employed by Amgen) and MNB (currently employed by Sanofi) are former employees of AstraZeneca and hold stock options in AstraZeneca. PD is a current contractor for and former employee of AstraZeneca and has previously held stock and/or stock options in the company. BRC is a consultant for AstraZeneca, Boehringer Ingelheim, Chiesi, Gala Therapeutics, GlaxoSmithKline, Menarini, Novartis, Pulmonx, and Sanofi-Aventis; is a data and safety monitoring board member for AstraZeneca, Sanofi-Aventis, and Vertex; is an advisory committee member for AstraZeneca, Chiesi, Gala Therapeutics, Menarini, Novartis, Pulmonx, Sanofi-Aventis, and Vertex; is a speaker for AstraZeneca, GlaxoSmithKline, and Menarini; and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events for AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, and Regeneron. CL, DH, SAB, and MAP have no disclosures to report.