Running Head: COPD Case Finding in Lung Cancer Screening
Funding Support: None
Date of Acceptance: August 25, 2025 | Published Online: August 29, 2025
Abbreviations: AUC=area under curve; AUROC=area under the receiver operating curve; CAPTURE=COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk; CAT=COPD Assessment Test; COPD=chronic obstructive pulmonary disease; CT=computed tomography; CTLS=CT lung cancer screening; FEV1=forced expiratory volume in 1 second; IQR=interquartile range; PEF=peak expiratory flow; SD=standard deviation; USPSTF=U.S. Preventive Services Task Force
Citation: Spetrini R, Pikman P, Kang V, et al. Prospective COPD case finding in a lung cancer screening program: a pilot study. Chronic Obstr Pulm Dis. 2025; 12(5): 411-418. doi: http://doi.org/10.15326/jcopdf.2025.0636
Introduction
A large proportion of patients with chronic obstructive pulmonary disease (COPD) remain undiagnosed, both in epidemiologic studies and at primary care settings.1 This is not unique to the United States, as the prevalence of COPD in 27 countries was 9.7% and of these, 81% were undiagnosed.2 This is a problem, because undiagnosed patients have similar impaired quality of life, reduced activities of daily living,3 and increased health care utilization, compared to those patients with a COPD diagnosis.4
Several factors have been identified as reasons for the underdiagnosis, including patient-related under-recognition and reporting of their symptoms, health care system-associated problems such as lack of access to spirometry, and lack of quality health care in middle- and low-income countries. Lastly, health care provider-related factors like poor understanding of diagnostic criteria and inadequate referrals to specialists also play an important role.5
The U.S. Preventive Services Task Force (USPSTF) recommends not screening asymptomatic individuals for the COPD diagnosis because of lack of evidence of any health care benefit when cases are detected.6 An alternative to find undiagnosed patients is to implement a case-finding strategy,7 which involves assessment of respiratory symptoms and disease risk factors before making a determination on whether or not further testing is required. One such strategy, using random telephone calls which involves the assessment of the presence of respiratory symptoms to offer spirometric screening of detection of airflow obstruction, has now been shown to improve health outcomes in patients with asthma and COPD.8
Active case finding involves the use of questionnaires to elicit respiratory symptoms in a target population at risk for COPD.5 Another technique is to use handheld devices that can measure peak expiratory air flow, thus facilitating a precise diagnosis of airway obstruction, particularly useful in resource-limited settings.9 Combined tools have shown a better diagnostic yield than either method alone.10 One such questionnaire, the COPD Assessment in Primary Care to identify Undiagnosed Respiratory Disease and Exacerbation Risk (CAPTURE) screening tool, is a 5-symptom and exposure questionnaire that is combined with peak expiratory flow (PEF) rate with sex specific thresholds to detect undiagnosed patients who may benefit from initiating therapy.11 The initial positive study involving this approach was tested in specialty clinics11 and subsequently in primary care practices.12 In this last context, a total of 4325 patients from primary care practices were enrolled and the study finally reported a sensitivity of 48% and a specificity of 89% (area under curve [AUC]: 0.81) to diagnose patients with clinically significant COPD. This low yield is perhaps explained by the fact that close to 50% of participants had no history of smoking and, hence, were unlikely to have COPD.
Undiagnosed COPD is quite prevalent in patients participating in a lung cancer screening program.13-15 However, there are limited reports of active case finding studies in this population, and none combining a symptoms questionnaire with a simple peak flow determination. We hypothesized that combining the CAPTURE questionnaire and PEF rate testing in a lung cancer screening population could be an effective strategy to detect patients with COPD, who could benefit from secondary and tertiary preventive measures. In addition, we also explored whether the presence of emphysema in the chest computed tomography (CT), completed as part of the lung cancer screening, performed differently than the peak flow meter cut-off in determining the presence of clinically significant COPD. This latter strategy would have value for those settings where access to a peak flow meter may not be available.
Methods
Study Design
This study was completed at Lahey Hospital and Medical Center, Burlington, Massachusetts. Patients were recruited between January 2023, and June 2024, if they were referred by a primary care provider from our institution to be enrolled in the chest CT lung cancer screening program (CTLS). We performed spirometry in 76 individuals, and 67 of them had acceptable spirometry quality and were included in the study. They had no prior diagnosis of COPD, had not performed a pulmonary function test (corroborated by reviewing our electronic medical record system), and were current or previous smokers with at least a 30-pack-year smoking history. Patients were excluded if they did not sign the consent form, did not complete the questionnaires, canceled the appointment, or were non-English speakers. The study was approved by the Lahey Hospital and Medical Center Institutional Review Board. All participants signed a consent form.
The Measurements
The day of the visit, participants completed a personal data form including anthropometrics, past medical history, and CAPTURE and COPD Assessment Test (CAT) questionnaires.11,16 The CAPTURE questionnaire was self-completed by all patients. The score ranges from 0 to 6, with higher scores reflecting higher exposure to polluted air, smoking or dirt; breathing changes with weather or air quality; breathing difficulty with activity; tiring easily compared to others of similar age; and missing work, school, or other activities due to bronchitis, pneumonia, or colds. The CAT questionnaire is a multidimensional 8-item questionnaire that assesses the health status of patients with COPD. The score ranges from 0–40. It is recommended that patients with a score >10 should be considered for regular treatment. Participants performed prebronchodilator spirometry following standard of practice17 using an Easy On spirometer (ndd Medical Technologies, Inc; Andover, Massachusetts) performed by study personnel trained in spirometry performance. The best of at least 3 maneuvers was reported. Spirometric values, including PEF values, were interpreted using the National Health and Nutrition Examination Survey prediction equations18 incorporated in the hand-held spirometer software for interpretation. Subsequent to this evaluation, a low-dose chest CT was completed. Patients and providers were informed of the results.
The definition of a positive screening included a CAPTURE questionnaire score of 5 or 6 or the combination of a score of 2–4 with a peak expiratory flow rate of <250 L/min for women and <350L/min for men. Besides the spirometric evidence of airflow limitation, clinically significant COPD was defined as patients with a CAT equal to or higher than 10. The presence or absence of emphysema was defined by the report from the blinded radiologist. Emphysema was quantified on the baseline screening CT scan as the percentage low attenuation area, defined as the percentage of lung volume with voxel density less than –950 Hounsfield units, using automated densitometry software (4DMedical; Carlton, Victoria, Australia). Data was stored in a secure server (REDCap; Nashville, Tennessee)
Statistical Analysis
The continuous variables were tested for the data distribution normality using the Shapiro-Wilk test. Normally distributed data were tested using unpaired Student T test and displayed as mean ± standard deviation. The skewed data were tested using Wilcoxon rank-sum test and displayed as median and interquartile range. The categorical variables were compared using Fisher’s exact test. The comparison with p ≤ 0.05 was considered significantly different. A receiver operating characteristic curve was generated for each criterion. The statistical analysis for this study was generated using Statistical Analysis Software version 9.4 for Windows (SAS Institute, Inc.; Cary, North Carolina).
Results
The clinical characteristics of the study patients are shown in Table 1. The 67 recruited patients had a mean age of 66 ±7 (y), 57% were female with smoking history of 37 (30–50) packs per year, 69% were ex-smokers abstinent for 14±10 year. A total of 27% of individuals screened had evidence of moderate airflow obstruction (forced expiratory volume in 1 second [FEV1] of 60±22% predicted), with significantly lower PEF rates and a numerically higher CAPTURE questionnaire score (2 versus 1). They were also more likely to have clinically significant COPD as demonstrated by a mean CAT score of 12 versus 7 for the non-COPD group. The Charlson comorbidity index was similar in both groups.
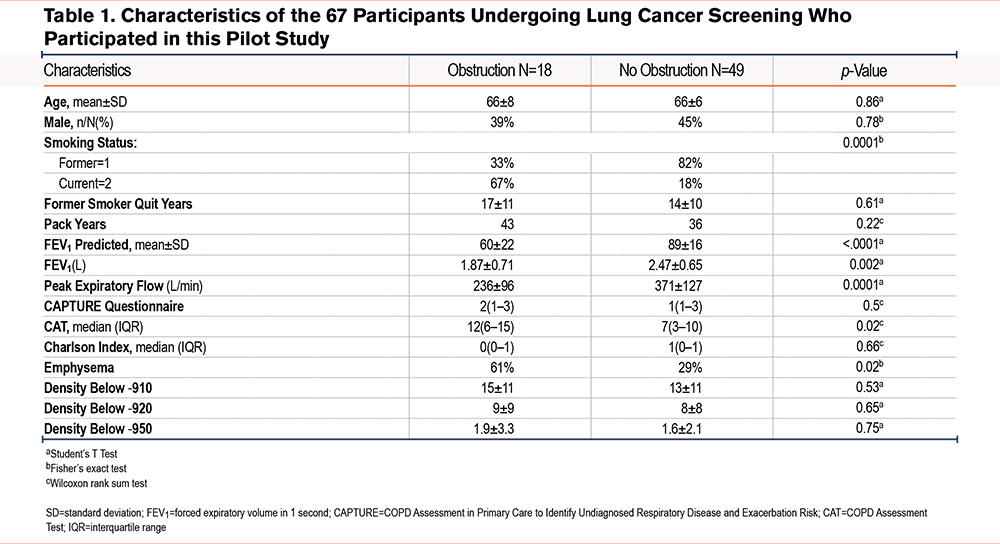
More patients in the COPD group had radiologic evidence of emphysema as assessed by the blinded radiologist reporting the chest CT scan (61% versus 29%). Further densitometry analysis showed a similar distribution in the upper, mid, or lower lung areas between obstructed and nonobstructed groups, as shown in Table 1.
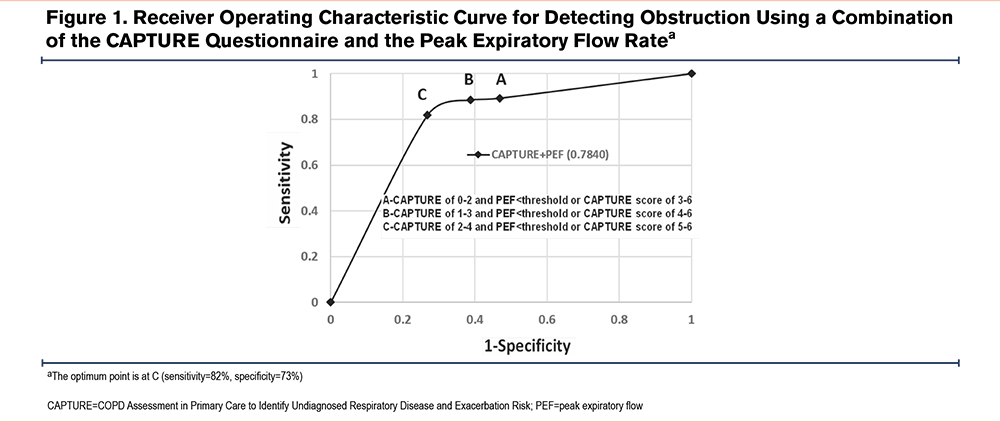
Figure 1 shows the receiving operator curve estimating the best point to describe the ideal combination of sensitivity and specificity to signal a COPD diagnosis. As seen in the figure, the combination of a CAPTURE questionnaire score of 2–4 combined with a PEF rate of <360L/min in men and <260L/min in women was able to diagnose clinically significant COPD with a sensitivity of 82% and a specificity of 73%. The AUC was 0.784. The combination of a CAPTURE questionnaire score of 2–4 combined with the presence of emphysema was able to diagnose clinically significant COPD with a sensitivity of 79% and a specificity of 73%. The AUC was 0.779 (results not shown). We compared best discerning points on both AUC curves and found no statistical difference in the capacity of both test combinations to diagnosed clinically significant COPD (McNemar’s test p= 1.000).
Discussion
This pilot study reports, for the first time, the performance of combining 2 different case-finding strategies for COPD diagnosis in a high-risk population of patients participating in a lung cancer screening program. Combining the scores of the CAPTURE questionnaire with a peak flow meter test helps select individuals likely to have clinically significant COPD. Additionally, using a CAPTURE questionnaire in combination with the presence of emphysema in a chest CT scan could serve as an alternative to performing a peak flow measurement, in sites where PEF testing may not be available.
COPD is an important health problem in the United States, with an estimated prevalence of approximately 13%; however, 71% of those persons remain undiagnosed, with little improvement in a 20-year period.19 Given the lack of evidence supporting the benefit of spirometric screening for COPD in the general population, the USPSTF has recommended against screening as a tool of any use to close this large diagnostic gap.6 However, case finding in selected populations, as is done for example in lung cancer screenings, does challenge the position of the USPSTF.7 Further, recent evidence provided by a large Canadian study indicates that by using the presence of respiratory symptoms determined via telephone calls, and screening those persons responding positively to the questions, it was possible to detect individuals with asthma or COPD. Importantly, the randomization of those detected sick persons to receive guided education and pharmacological therapy compared with usual care, was effective in improving health-related outcomes.8 Unfortunately, the methodology used in this study, random digit dialing, is relatively expensive and inefficient.
One potential approach to improve COPD case finding has been the development of questionnaires to detect people likely to have the disease. One such questionnaire, the CAPTURE, has received significant attention because of its careful development and validation.11 However, in a large study conducted in the primary care setting, its performance failed to replicate the positive results of the original reports.12 Of note, the majority (58%) of individuals included in the study were never smokers and only 12% had a history of active smoking, making them unlikely to have obstruction to airflow.12 Therefore, it is not surprising that more efforts are required to close the gap between patients with spirometry diagnosis of COPD and those who remain symptomatic but undiagnosed.
Tobacco smoking is the most important cause of COPD in susceptible individuals in the United States. Therefore, studying a population with higher tobacco use should be more efficient to find undiagnosed patients with COPD than screening a general population. The criteria for lung cancer screening requires participants to be current or former smokers, with an age and smoking history where it is likely that individuals susceptible to cigarette smoke would have obstruction to airflow and symptomatic COPD. This may help explain why our study had a higher sensitivity (82%) compared to the 42% reported by Martinez et al in the primary care setting.12 Our population had a 100% history of tobacco use with a minimum of 30-pack-year history compared to 42% reporting ever using tobacco in the study by Martinez et al.
Another important finding in this pilot study is the potential use of the presence of emphysema as assessed by CT scans in helping select individuals likely to have COPD. This finding could positively impact the implementation of screening tools in CTLS programs, where performance of a peak flow measurement could be difficult due to reduced resources and lack of personnel able to perform the PEF measurement. A CAPTURE questionnaire could be provided to all participants and those with 2 or more positive questions and presence of emphysema on the CT could be referred to pulmonary function testing. The presence of emphysema coupled with a CAPTURE questionnaire score of 2–4, has a similar sensitivity and specificity than the use of the CAPTURE tool alone, with a nonstatistical different AUC. This selection process would also reduce the number of participants in a CTLS program who need to perform a pulmonary function test. As we previously showed, the presence of emphysema should be part of the standard CTLS report. Its presence has been associated with increased risk of COPD-related hospitalizations.20 This information is also in line with the Lancet Commission on COPD that suggests to move beyond the reliance on spirometry to identify patients with obstructive lung disease.21
The diagnosis of COPD has also been shown to positively affect tobacco cessation.22 We can speculate that an additional benefit of finding COPD patients in a CTLS program is to advise them of their higher likelihood of developing lung cancer in the future. As previously reported by de Torres et al, patients with a COPD diagnosis and evidence of emphysema have up to 3.5 times higher risk of developing lung cancer23-25than individuals without those findings, particularly among women and those with milder obstructive disease.
Our study has several limitations. First, it was performed in a single center with a well-established CTLS program. However, we believe this simple approach to COPD diagnosis could be replicated in other programs combining the use of the CAPTURE questionnaire in all participants being screened and performing a peak flow maneuver instead of spirometry, or else combining the questionnaire and the presence of emphysema. We believe the substantial yield justifies the effort. Second, we enrolled a small number of participants compared to previous studies using the CAPTURE questionnaire and peak flow meter measurements.11,12,26 However, we showed significantly positive screening results in identifying patients with clinically significant COPD who would benefit from intervention27 with a higher yield (sensitivity and specificity) compared to larger studies completed in primary care settings.12 A positive result in a relatively small number of participants supports an important clinical value. Third, we do not know if the COPD diagnosis influences patient behavior and treatment, as this study was not planned to follow patients after the intervention to determine if tobacco cessation and bronchodilator therapy prescription was implemented. However, further intervention studies are urgently needed given the positive results of the case-finding study in symptomatic patients recruited using telephone calls in Canada, reported by Aaron et al.8 Fourth, a larger sample may allow us to perform further analysis regarding the emphysema severity and location and its relationship to the COPD diagnosis, and the clinical implications of different emphysema distribution.20
In summary, we demonstrated that the use of the CAPTURE questionnaire combined with peak flow meter measurement is an effective tool to diagnose clinically significant COPD. The presence of emphysema on the chest CT could be used in CTLS programs where the peak flow measurement might be difficult to obtain. Case finding in persons participating in lung cancer screening programs should help close the large underdiagnosis gap of COPD currently present in the United States.
Acknowledgments
Author contributions: PP, VK, LG, YL, AC, BC, and VPP contributed to the conception and design of the study. RS, PP, VK, JB, HR, KA, NA, and PO assisted with the acquisition of data. YL, LG, BCC, and VPP provided statistical analysis support. YL, LG, BCC, and VPP were the primary composers of the manuscript. All authors contributed to the data interpretation as well as to the editing and final approval of the manuscript.
Declaration of Interest
RS, PP, VK, JB, HR, KA, NA, PO, KC, LG, YL, AC, RT, and VPP have no conflicts of interest to declare. BC is a consultant for AstraZeneca, Boehringer Ingelheim, Chiesi, Gala Therapeutics, GlaxoSmithKline, Menarini, Novartis, Pulmonx, and Sanofi-Aventis; is a data and safety monitoring board member for AstraZeneca, Sanofi-Aventis, and Vertex; is an advisory committee member for AstraZeneca, Chiesi, Gala Therapeutics, Menarini, Novartis, Pulmonx, Sanofi-Aventis, and Vertex; is a speaker for AstraZeneca, GlaxoSmithKline, and Menarini; and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events for AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, and Regeneron.