Running Head: Polypharmacy in Patients With COPD
Funding Support: None
Date of Acceptance: August 13, 2025 | Published Online: August 29, 2025
Abbreviations: ADRs=adverse drug reactions; CI=confidence interval; COPD=chronic obstructive pulmonary disease; MLTCs=multiple long-term conditions; mMRC=modified Medical Research Council dyspnea questionnaire; OR=odds ratio; PRISMA=Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Citation: Upadhyay H, Akter F, Koumides A, Husband A, DeSoyza A. Polypharmacy in patients with COPD: a scoping review. Chronic Obstr Pulm Dis. 2025; 12(5): 440-449. doi: http://doi.org/10.15326/jcopdf.2025.0630
Online Supplemental Material: Read Online Supplemental Material (201KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a global health challenge.1 The Global initiative for chronic Obstructive Lung Disease (GOLD) report recommends a combination of pharmacological and nonpharmacological therapies to manage COPD.2 The focus for current pharmacological therapy is based on symptoms and disease severity markers such as pulmonary exacerbations, with more recent guidance also linked with blood eosinophil levels. Pharmacotherapy includes inhaled bronchodilators and corticosteroids depending on the above-mentioned factors. COPD medications are often administered through various inhaler devices, which have the potential to cause issues with inhaler technique, adherence, and treatment burden.2 In addition to inhaled regimens, more severe COPD may require additional treatments such as roflumilast and/or prophylactic antibiotics. Therefore, COPD management, even in isolation, can be associated with polypharmacy. Those patients on multiple medications have a high risk of suffering from adverse drug reactions (ADRs).2
Polypharmacy has various definitions in literature, depending on the number of medications prescribed. A systematic review of definitions done by Masnoon et al explored various terms used in existing literature. This review included 110 studies, out of which 57 studies (51.8%) defined polypharmacy or major polypharmacy as the use of ≥5 drugs, minor polypharmacy as use of 0 to 4 drugs (8 studies [7.3%)]), and moderate polypharmacy as the use of 4 to 5 drugs (1 study [0.9%]). The use of ≥10 drugs was defined as hyper polypharmacy (1 study [0.9%]), excessive polypharmacy (7 studies [6.4%]) or severe polypharmacy (1 study [0.9%]).3
Multimorbidity in the general population is associated with polypharmacy and specifically a diagnosis of COPD is associated with an increased risk of polypharmacy.4 Multimorbidity is defined as the coexistence of 2 or more multiple long-term conditions (MLTCs), without reference to a primary condition, that may overlap, vary in severity, and change in terms of health burden over time.4,5 COPD is associated with multimorbidity with higher rates of cardiovascular diseases (congestive heart failure, ischemic heart disease, arrhythmias, peripheral vascular disease, hypertension, and atrial fibrillation) as well as bronchiectasis, sleep apnea, anemia, secondary polycythemia, lung cancer, diabetes, osteoporosis, anxiety/depression, and gastroesophageal disease.2 These may significantly impact the patient’s clinical condition and prognosis. These conditions can also mimic the symptoms of COPD, limit lung function, or complicate the management of COPD.2
Patients with COPD are therefore more likely to have MLTC, polypharmacy, risks of ADRs, hospital re-admissions, and premature mortality.2,4,5
Aim
This review aims to report the prevalence of polypharmacy, the varying definitions of polypharmacy used within COPD populations, factors associated with polypharmacy, and medication-related harms in COPD patients.
Methods
Overview
A scoping review was selected to consolidate knowledge on polypharmacy in COPD populations. We identified that evidence in this topic required investigation, and a scoping review enables key findings to be quickly summarized and disseminated. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines for scoping reviews: (1) identify objectives; (2) identify relevant studies; (3) analyze the literature for relevance; (4) extract key data; and (5) review and report results. A prespecified protocol was used to guide data synthesis. The protocol was registered through the Open Science Framework,6 ensuring transparency and adherence to the established objectives and methodologies for scoping reviews.
Identifying Objectives
The following main objectives were identified as being key areas of interest: What is the prevalence of polypharmacy in COPD? What are the varying definitions of polypharmacy used within COPD populations?
Identifying Relevant Studies
Search Strategies and Databases
A systematic literature search of the electronic databases PubMed, Medline, and Embase was conducted using Medical Subject Headings terms and keywords identified from various systematic and scoping reviews relevant to the topic.
The first search was executed in PubMed on January 15, 2024, and the last one on Medline on January 24, 2024. A finalized search strategy was formulated by assessing the number and quality of returns, followed by a literature search executed on January 26, 2024, across 3 databases: PubMed, Medline, and Embase (Supplementary Table S1 in the online supplement).
A secondary search conducted in PubMed on February 6, 2024 (Supplementary Table S2 in the online supplement) targeted systematic reviews examining polypharmacy, enhancing the methodological rigor by identifying and incorporating established methods from existing reviews. Acknowledging that eligible studies might be indexed under more general terms not relating to COPD, another secondary search was performed in PubMed on February 10, 2024, focusing on polypharmacy by incorporating terms identified from other systematic and scoping reviews (Supplementary Table S2 in the online supplement).
Eligibility Criteria
The inclusion criteria for this scoping review were as follows: (1) Studies that clearly defined their COPD population, and (2) Studies that contained data on the prevalence of polypharmacy that was reported explicitly for a COPD cohort.
The exclusion criteria for this scoping review were as follows:
- Studies in languages other than English;
- Studies that were aimed at pulmonary diseases other than COPD;
- Studies that did not mention polypharmacy in patients with COPD;
- Studies that did not detail the rates of polypharmacy in their study population;
- Animal and genetic studies;
- Conference abstracts and nonpeer-reviewed articles.
Analyzing the Literature for Relevance
Duplicate studies were removed using EndNote (IBM Systems; Armonk, New York). Two independent reviewers assessed each abstract and formulated a list of “relevant” and “of uncertain relevance” studies. The full manuscripts that met either of these criteria were subsequently independently reviewed by the 2 reviewers, and eligible studies were identified. When consensus was not met, a third independent reviewer adjudicated.
Data Extraction
A charting form was developed for data extraction. A single reviewer independently extracted the following data from eligible studies: title, author, date of publication, location, study type, methods, study size, total COPD patients in the cohort, care center, study period, definition of polypharmacy used in the study, and reported prevalence of polypharmacy. The care center details were extracted from the eligible studies and as each country used different definitions to identify primary/secondary/specialist centers, it was not possible for the authors to standardize these definitions.
We recorded the polypharmacy rates according to the original sources and also, where possible, within the definitions of polypharmacy as the use of ≥5 medications and hyper polypharmacy as the use of ≥10 medications as proposed in a systematic review by Masnoon et al.3
Results
The initial database search identified 2573 articles, and 28 studies met the inclusion criteria. The number of studies omitted at each stage and the reasons for exclusion are described in Figure 1.7
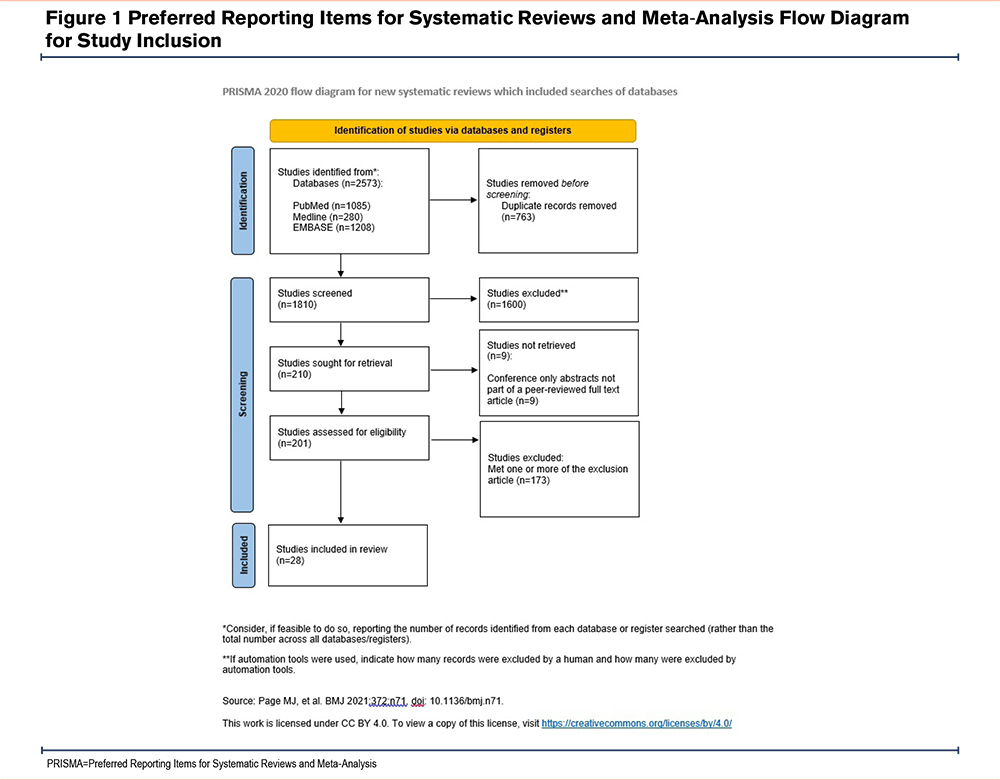
The data was extracted from the 28 eligible articles and has been summarized in Table 1.4,5,8-33 Some papers did not report values for all characteristics, and this is reflected in the table.
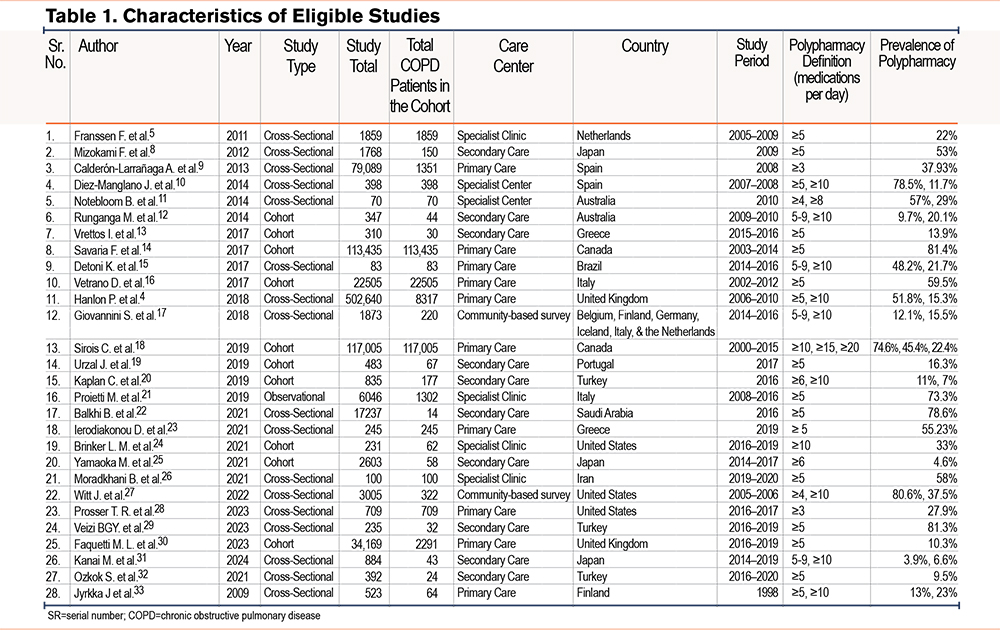
Study Characteristics
Table 1 presents an overview of the characteristics of the 28 included studies, including author details, year of publication, study design, total patients with COPD, type of care center, country of publication, study period, definition of polypharmacy used in the study, and prevalence of polypharmacy.
A total of 3 studies each were carried out in the United States (10.7%), Japan (10.7%), and Turkey (10.7%), followed by 2 studies each in the United Kingdom (7.1%), Spain (7.1%), Australia (7.1%), Canada (7.1%), Greece (7.1%), and Italy (7.1%). One study each was conducted in the Netherlands (3.6%), Brazil (3.6%), Portugal (3.6%), Saudi Arabia (3.6%), Iran (3.6%), and Finland (3.6%). One study (3.6%) was conducted across multiple centers in Europe in Belgium, Finland, Germany, Iceland, Italy, and the Netherlands.
A variety of study designs were reported, including cross-sectional (17, 60.7%), cohort (10, 35.7%), and observational (1, 3.6%). The settings included secondary care (10, 35.7%), primary care (10, 35.7%), specialist clinic (6, 21.4%), and community-based surveys (2, 7.2%).
Study Population
The number of patients with COPD in the 28 articles ranged from 14 to 117,005.
Collectively, the studies had a total population of 909,079 patients, out of which 270,977 (29.8%) patients had COPD. Out of 28 studies, 11 studies (39.3%)5,10,11,14-16,18,21,23,26,28 consisted of only patients with COPD, while the rest of the 17 studies4,8,9,12,15,17,19,20,22,24,25,27,29-33 had mixed COPD and non-COPD cohorts. However, in all 28 studies, the polypharmacy rates for patients with COPD were extractable.
Definition of Polypharmacy
The majority of the studies (17, 60.7%) used a single threshold to define polypharmacy, whereas, the remaining 11 studies classified polypharmacy into varying subgroups. The most common single threshold was ≥5 medications (13, 46.3%), followed by ≥3 medications (2, 7.1%), ≥6 medications (1, 3.6%), and ≥10 medications (1, 3.6%).
The most common subgroup classification seen was the use of 5–9 and ≥10 medications (4 studies, 14.3%). Three studies (10.8%) classified patients into 2 groups: those using ≥5 and ≥10 medications. One study (3.6%) classified patients who used ≥10, ≥15, and ≥20 medications; 1 study (3.6%) defined polypharmacy as ≥4 and ≥8 medications, followed by use of ≥4 and ≥10 medications (3.6%), and the remaining study (3.6%) classified polypharmacy as the use of ≥6 and ≥10 medications (Table 1).
Polypharmacy and hyper polypharmacy rates were varied across literature. Polypharmacy rates in studies that used a single threshold of ≥5 medications ranged from 9.5% to 91.4%. Studies that classified polypharmacy in subgroups of 5–9 and ≥10 medications had polypharmacy rates ranging from 3.9% to 12.1% and 6.6% to 21.7% respectively. Similarly, polypharmacy rates in subgroups of ≥5 and ≥10 medications ranged from 13% to 78.5% and 11.7% to 23%, respectively.
Factors Associated With Polypharmacy
The most common factors associated with polypharmacy were older age, increasing number of comorbidities, and smoking status.10
Age
Twelve studies reported that polypharmacy increased with age, typically reporting the significance of the increase was in ages 65 and over.4,8,10,13,14,16,18,23,24,30-32 Certain studies showed a statistically significant association,8,13,14,16,23,27,31,32 however, many other studies4,10,18,24,30 did not report the statistical association. Most studies showed a strong link with polypharmacy and age, with one study showing a higher rate of polypharmacy in older patients (86.8% among patients aged ≥75 years),14 while 2 studies showed no links between age and polypharmacy.5,19 Surprisingly, a cross-sectional study across multiple European countries in home care patients showed an inverse association of hyper polypharmacy with age (odds ratio [OR] 0.69, 95% confidence interval [CI] 0.56–0.83).17
Gender
Gender had a significant role to play with polypharmacy status. A total of 4 studies showed a higher prevalence of polypharmacy in females, 8,17,32,33 while 2 studies showed a higher prevalence in males.9,23 One study showed no statistically significant difference between polypharmacy and gender.5
Comorbidities
Comorbidities and polypharmacy were common phenomena reported in 13 studies.4,10,13,14,16-18,23,24,30-33 The most common diseases included diabetes mellitus,4,10,13,14,18,24,30,31 cardiovascular diseases,4,10,13,14,18,23,24,31 dyslipidemia,13,24,31 cerebrovascular diseases,13,14,31 dementia,13,31 and COPD,4,10,13,16,23,24,31 which is relevant to our study. A total of 3 studies4,23,24 also highlighted the increasing risk of polypharmacy in patients with depression-anxiety diagnosis.
Smoking Status
COPD patients with polypharmacy were more frequently ex-smokers as compared to nonsmokers (75.4% versus 68.4%; p=0.0005).10 The average pack years between those with and without polypharmacy was significantly different (58 versus 40 years, p=0.004).23
Medication Adherence
Polypharmacy was linked to poor medication adherence only in COPD patients aged ≥65 years (OR 1.34, 95% CI 1.13–1.59).16 Poor adherence with polypharmacy (p<0.001) was also seen in an observational study done by Moradkhani et al.2 A regression model was run in this study which showed the odds of patients with polypharmacy having high medication adherence was 81% less than those without polypharmacy, while controlling for other covariates (p = 0.008).26
COPD patients were more likely to have polypharmacy (73.3% to 55.6%, p value <0.001), and the combination of COPD and polypharmacy led to poor medication adherence.32 Vetrano et al reiterated in their study how complex regimens lead to poor adherence.16
Health Status and Frailty
In addition to poor functional health status, polypharmacy patients were less likely to be living independently (p=0.017).27 This may correlate with studies suggesting higher rates of frailty in these group as the Charlson Comorbidity Index, which predicts the 10-year mortality in comorbid patients, was used by Díez-Manglano et al10 to determine that polypharmacy was associated with a worse Charlson score (p=0.004).
Polypharmacy was significantly associated with failure to improve activities of daily living in a mixed cohort of COPD and non-COPD patients (OR 2.22. 95% CI 0.48–10.16).12
Two studies25,32 reported that polypharmacy patients were at a higher risk of pain, falls, dyspnea, and flare-ups. When asked, polypharmacy patients self-report poor health status as well.33
Hospital Admissions
Díez-Manglano et al reported an increase in medications even after hospital admission for COPD acute exacerbation, with the mean number increasing from 5 to 6.6 at discharge.10
The IBenC study by Giovannini et al1 in home care patients (including those with COPD), showed that recent hospitalization in the past 90 days was statistically associated with increased probability of hyper polypharmacy (P-value 0.012).
Severity of COPD
Franssen et al reported increasing use of respiratory drugs with advancement in GOLD stages and a worsening modified Medical Research Council (mMRC) dyspnea score.5 An observational study done by Díez-Manglano et al showed polypharmacy was associated with severe dyspnea using the mMRC scale (3.7 versus 3.5, p=0.02), however, patients with polypharmacy showed no differences in their GOLD staging as compared to nonpolypharmacy patients.10
Multimorbidity
A cross-sectional study in Greece found the majority of the patients with polypharmacy were suffering from multimorbidity as compared to those without polypharmacy (98% versus 51%, p<0.001).2
Clinical Outcomes of Polypharmacy
Most extracted studies provided little information on how polypharmacy was linked to ADRs, hospitalization, and mortality in a COPD-specific population. A cross-sectional UK biobank study in 8317 patients with self-reported COPD reported higher risk of ADRs, such as bleeding (OR 4.61, 95% CI 3.35 to 6.19), CNS depression (OR 3.75, 95% CI 3.31 to 4.25), constipation (OR 3.42, 95% CI 3.10 to 3.77), urinary retention (OR 3.38, 95% CI 2.94 to 3.87), falls (OR 2.27, 95% CI 2.13 to 2.42), and renal injury (OR 2.22, 95% CI 1.86 to 2.62).4
Similarly, a cross-sectional study from Greece showed that COPD patients with polypharmacy were at a higher cumulative risk of falls, constipations, and cardiovascular events. In addition, 15 pairs of major drug‐to‐drug interactions were recorded in 11.5% cases, however, the details of these were not mentioned.23
Additionally, Witt et al reported, in a cross-sectional study of 3005 U.S. community-dwelling older adults, high-risk medications increased ADRs, and this risk furthered with polypharmacy. These high-risk medications included antihistaminic, anticholinergic, benzodiazepines, antipsychotics, anxiolytics/sedatives, tricyclic antidepressants, muscle relaxants, anti-arrhythmic, COX-2 inhibitors, and narcotics.27
Detoni et al conducted a retrospective review of medication management services delivered to COPD patients and identified the drug therapy problems in these patients. The most common drug therapy problem in COPD patients was ADR (25.8%), followed by unnecessary drug therapy (20.8%), and nonadherence to prescribed medications (18.8%). The most common ADR identified was oral candidiasis associated with the use of inhalers, however, the frequency of the ADRs were not described.15
Discussion
Despite the increasing prevalence of COPD worldwide, polypharmacy in COPD is poorly understood and described in literature. Our literature search identified 28 studies that reported the prevalence of polypharmacy in patients with COPD, however, there was no set definition for polypharmacy across these papers. Most studies defined polypharmacy with a threshold of ≥ 5 medications,5,8,13,14,16,19,21-23,26,29,30,32 but other studies defined polypharmacy as the use of ≥3 medications,9,28 ≥6 medications,25 and ≥10 medications.24 A further subcategory was defined in 7 papers wherein hyper polypharmacy was studied along with polypharmacy.4,10,12,15,17,31,33 These varied definitions create inconsistencies across studies and limit the conclusions that can be drawn from these studies. Allowing for this, the studies suggest varied polypharmacy rates ranging from 9.5% to 91.4% in studies with a single definition of polypharmacy (≥ medications). Similarly, studies with polypharmacy subgroups of 5–9 and ≥10 medications had polypharmacy rates ranging from 3.9% to 12.1% and 6.6% to 21.7%, respectively. This large range likely reflects both the different patient populations and settings as well as the varying definitions.
COPD therapy often involves the use of combination inhalers, e.g., triple inhaled long-acting beta agonist, long-acting anticholinergic, and inhaled corticosteroid in a single inhaler. Consensus is needed on how best to report these in polypharmacy studies as the use of triple therapy can be classified as either the use of 3 different medications or one single inhaler. Drug-drug interactions are defined by the number of active pharmaceuticals taken, but compliance may be different in those with simpler drug regimens as compared to those with “open triple” and “multiple inhalers.”
Overall, we found the most common factors associated with polypharmacy were older age, increased comorbidities, and smoking status.4,5,9,10,14,17,23,27,32,33 Some studies showed polypharmacy associated with increased age with several studies reporting a statistically significant association.8,13,14,16,23,27,31,32
However, COPD is commonly associated with MLTC, and both MLTC and COPD prevalence increases with age. The various methodologies used in the extracted literature make it difficult to understand what are independent predictors of polypharmacy. Notably, polypharmacy and comorbidities both contribute to poor adherence to medication in patients with COPD.13,16,32
Comorbidities are also common in patients with COPD, with the most common ones being cardiovascular diseases. COPD-heart failure and COPD-coronary artery disease clusters are strongly associated with polypharmacy.8,10 Apart from cardiovascular diseases and COPD, polypharmacy was also associated with diabetes mellitus and dementia.13
These comorbidities are drivers to polypharmacy, increasing the use of potentially inappropriate medications, medication error, poor drug adherence, drug-disease interaction, and drug-drug interactions that can lead to severe ADRs.9,10,21
Polypharmacy is a concern not only due to the immediate costs of the medication regimens and the increased risk of nonadherence (which in turn increases the risk of COPD exacerbations), but also due to the concern over ADRs and drug-drug interactions.15 A major proportion of COPD health care costs relate to hospitalizations. Notably, hospitalized patients with COPD are twice as likely to have polypharmacy regimens than non-COPD hospitalized patients.24 The available literature, however, is limited in looking at the independent contributions of polypharmacy, frailty, and multimorbidity in driving hospitalizations. Relatively few studies do comment on potential adverse drug reactions in COPD as possible factors associated with admission.23 Ierodiakonou et al found specific drug-drug interactions, such as liver damage, rhabdomyolysis, and arrhythmia, increased with polypharmacy, suggesting side effects of multiple medications being a cause for increased hospital admission.23
Detoni et al investigated the most common types of medication-related problems in a cohort of 83 patients with COPD and non-COPD patients in Brazil and found that ADRs were the most common (25.8%), with unnecessary medication use (defined as duplicate therapy, no medical indication, or where nonmedication therapy was more appropriate), and nonadherence the second and third most common problems respectively (20.8% and 18.8%, respectively).15 High levels of inappropriate medication use in patients with COPD suggests the need to emphasize and screen for potentially inappropriate medications in these patients to help reduce medication burden, improve adherence, and reduce the risk of hospitalization. One of our stated aims was to report the medication-related harms in COPD, but we found the literature lacking in this detail. Therefore, future prospective studies should consider this in their study design.
Since polypharmacy is associated with a high risk of ADRs, drug-drug interactions, and reduced medication adherence, specialist pharmacy services can be utilized in respiratory clinics to reduce the impact of polypharmacy and provide regular medication review to patients. In addition, COPD is associated with multimorbidity (especially cardiovascular diseases) which is often managed by specialists or primary care physicians, hence, a high degree of care coordination and effective communication between multidisciplinary teams will improve patient outcomes.
A few studies we identified have analyzed polypharmacy in hospitalized elderly patients wherein polypharmacy was more common in those 75 years or older and with multiple comorbidities.8,17 These patients with polypharmacy are at increased risk of frailty and worse long-term outcomes.9,12 In terms of smoking status and medication adherence, nonsmokers were more compliant with long-term treatment than smokers and ex-smokers.16
Future studies require attempts to avoid unhelpful variation in definitions. It is commonly held that polypharmacy is defined as the use of ≥ 5 medications and hyper polypharmacy is ≥ 10 medications. These will usefully be applied in future studies as they are aligned with current thinking. However, future studies should also consider analyses where drug count is also analyzed using continuous statistical approaches beyond the current categorical approach. Future studies would be advised to be methodical in reporting drug regimens as the complexities of COPD pharmacotherapy with open and closed triple inhalers needs critical thought. Closed triple inhaled therapy represents a reduced treatment burden, for example, and the potential for drug-drug interactions may be different from that where patients are variably complying with open triple therapy. Future studies should state if COPD was diagnosed by spirometry and ideally, these studies should compare patients who were solely managed by primary care and those who were hospitalized or had received outpatient hospital-based COPD care.
Future work with a clear definition for polypharmacy is needed with more cross-sectional and longitudinal studies of polypharmacy in people living with COPD. Better clarity on the independent predictors of hospitalizations is also needed, and these studies should consider the complex interactions between adherence, MLTC and frailty, polypharmacy, and age. Studies that highlight the patient factors and medication combinations that enhance the risk of adverse medication reactions are needed. Additionally, prospective studies should consider analyses by sex and smoking status. Interventional studies to rationalize medication regimens are also welcomed.
One of the limitations of the study was that the available literature notes a diagnosis of COPD, but the studies herein rarely, if ever, documented if this was a spirometry supported COPD diagnosis. In addition, the available literature does not allow us to make recommendations of suitable interventions to minimize treatment burdens or risk of drug-drug interactions.
Conclusion
Polypharmacy in COPD patients is common yet poorly understood due to difficulty in comparing previous literature with differences in methodologies, patient populations, and definitions of polypharmacy. The most common factors associated with polypharmacy were old age, number of comorbidities, and smoking status. There is likely a complex interaction between the multimorbidity present within a COPD population that amplifies risks of polypharmacy. Polypharmacy is a growing concern due to increasing costs, nonadherence to medications, ADRs, and drug-drug interactions. Clinicians should be mindful of the patient’s age, comorbidities, and risks of drug-drug interactions while prescribing medications in the COPD clinic.
Acknowledgments
Author contributions: HU, FA, and AK contributed to the acquisition and analysis of data. AH and AD contributed to the conception and design. All authors drafted the initial version of the manuscript, and AD and AD reviewed the initial version of the manuscript. All authors gave final approval of the manuscript.
Declaration of Interest
In the past 36 months, HU has received funding from the National Institute for Health and Care Research Clinical Credentials Framework to undertake a postgraduate certificate in research. In the past 36 months, ADS has received grants from Boehringer Ingelheim, Sanofi, Innogen, AstraZeneca, GSK, and Inogen; has received consulting fees from Insmed, Fisher and Paykel, Sanofi, 30T, AstraZeneca, and GSK; has received honoraria from Insmed, Fisher and Paykel, Sanofi, Innogen, AstraZeneca, and GSK; has received support for attending meetings and/or travel from Sanofi and has participated on a data safety monitoring board/advisory board for Bayer. All other authors have nothing to declare.