Running Head: BMI and Exercise Improvements in AATD Individuals
Funding Support: This study was funded by an unrestricted research grant from AlphaNet.
Date of Acceptance: August 13, 2020 | Published Online Date: October 30, 2020
Abbreviations: Step Forward Study, SFS; body mass index, BMI; alpha-1 antitrypsin deficiency, AATD; standard deviation, SD; chronic obstructive pulmonary disease, COPD; alpha-1 antitrypsin, AAT; AlphaNet Disease Management and Prevention Program, ADMAPP; virtual pulmonary rehab, VPR; Body mass index-airflow Obstruction-Dyspnea-Exercise capacity, BODE
Citation: Choate R, Mannino DM, Holm KE, Beiko T, Boyd B, Sandhaus RA. Home-based multicomponent intervention increases exercise activity and improves body mass index: results of a 5-year randomized trial among individuals with alpha-1 antitrypsin deficiency-associated lung disease. Chronic Obstr Pulm Dis. 2021; 8(1): 7-18. doi: http://doi.org/10.15326/jcopdf.8.1.2020.0183
Online Supplemental Material: Read Online Supplemental Material (1351KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is a genetic condition that increases the risk of developing lung disease, particularly the emphysema subtype of chronic obstructive pulmonary disease (COPD). Among patients with AATD-associated lung disease, management options include usual COPD therapy, timely start of augmentation therapy with purified alpha-1 antitrypsin (AAT) infusions, and health maintenance that includes exercise and a proper diet.1 Physical activity is extremely important for individuals with lung disease. Individuals with COPD who are less physically active have worse quality of life, more hospitalizations, and a shorter lifespan.2 It is also important for individuals with lung disease to maintain a healthy body mass index (BMI), given that both extremes of BMI are associated with worse outcomes among patients with COPD. For example, low BMI is associated with mortality3-5 and worse quality of life,6,7 and high BMI is associated with increased health care utilization8 and worse quality of life.7
AlphaNet, a not-for-profit organization founded in 1995 by patients with AATD, developed the Alpha-1 Disease Management and Prevention Program (ADMAPP) to empower patients with AATD to improve their quality of life and health outcomes. ADMAPP includes comprehensive patient self-education and provides various resources for patients, including regular telephonic interactions with trained coordinators (also diagnosed with AATD).9,10 One of the foundational elements of ADMAPP is the “Alpha-1 Big Fat Reference Guide,” which includes essential information about the disease, management recommendations, guidance regarding health care visits, and therapies as well as general information about living with AATD.10
The effectiveness of this disease management program has been evaluated by comparing outcomes in the year prior to the introduction of the program to outcomes following implementation of the program in 1000 AATD lung-affected individuals.10 Results showed significant improvements in disease-specific and general quality of life, improved health outcomes, and increased knowledge about the disease. Based on these results, this program is now considered standard care and was adopted for all AATD patients followed by AlphaNet. While the disease management program was associated with improvements in multiple outcomes, participation in this program was not associated with an increase in exercise activity and over 60% of participants were overweight or obese (defined as BMI ≥ 25).10
In response to the findings regarding exercise activity and BMI, AlphaNet developed the Step Forward Study (SFS) to examine the effect of an intensive distance intervention delivered via a series of teleconferences, videos, and mailings and designed to increase exercise activity and improve BMI. The primary outcome was self-reported number of exercise minutes per week, and the secondary outcome was BMI change. All participants continued participating in AlphaNet’s disease management program designed for individuals with AATD-associated lung disease who have been prescribed augmentation therapy. It was hypothesized that an intensive distance intervention along with the standard care provided through ADMAPP will increase exercise activity and improve BMI compared to the standard care alone.
Methods
Study Design
The SFS is a prospective, double-blind randomized, interventional study designed to examine the effectiveness of an intensive distance intervention in increasing exercise activity and improving BMI over time. This study was approved by Western Institutional Review Board (approval #1107247). Individuals who met the eligibility criteria outlined in Supplemental Table 1, in the online supplement, were invited to participate and provided written, informed consent.
Randomization
Upon receipt of a signed consent form, participant data were entered into a secure database. Based on sequential database entry numbers, the participants were randomized 1:1 with odd identification (ID) numbers to receive standard care and even ID numbers to receive standard care plus the intensive distance intervention. The specific method of randomization and information regarding treatment assignment were not available to the principal investigator, the study personnel or the study participants. In addition, the analytic team members were blinded until data analysis was complete. AlphaNet coordinators, also individuals with AATD who had undergone special training and became the main points of contact for all individuals followed by AlphaNet, were not blinded and played an important role in the execution of the study and data collection.
Intervention
Throughout the study period, participants in both groups continued participating in AlphaNet’s standard disease management program.10 To create and maintain blinding with regard to treatment assignment, all participants (including those in the standard care group) received study-specific materials including general educational materials, pedometers, harmonicas, and assessment tools as outlined in Supplemental Table 2 in the online supplement.
Participants assigned to the intervention group were mailed 7 sets of intensive intervention activities (e.g., elastic exercise bands, exercise balls, virtual pulmonary rehab [VPR], etc.) throughout the study period as highlighted in the Supplemental Table 2 in the online supplement. The intensive interventions were tailored according to the baseline BMI of the participants to improve their weight status: to increase body weight in those in the low BMI category, maintain weight in the normal category, and reduce body weight in the high and very high BMI categories. The interventions were added cumulatively between October 2009 and November 2013. Timings and description of these mailings are presented in the Supplemental Table 2 in the online supplement. Pictures of the selected mailings and a detailed description of the VPR materials are presented in the Supplemental Figures 2 and 3 in the online supplement.
Outcome data were collected over a 5-year period beginning in October 2009 and ending in August 2014.
Measures
Exercise activity. Participants were instructed to record daily minutes of 3 types of exercise: warm-up, cardio, and strength. Exercise minutes were recorded in diaries provided by AlphaNet. These diaries also captured information regarding weight, pedometer activity, and meal sizes. Each diary page was designed to capture 7 days of data, and participants were instructed to enter information on a daily basis (Supplemental Figure 1 in the online supplement). Overall daily exercise activity was calculated as the sum of all 3 exercise types. All analyses for exercise activity used minutes of exercise activity per week, which was calculated as the sum of overall daily exercise activity for all days in a week. In participants with no exercise activities entered for any day in a given week, the exercise values for the respective week were set as missing. High outliers of weekly exercise activity minutes were identified as 3 standard deviations from the mean and conservatively limited to that value. In individuals with exercise minutes with zero values entered for weeks when measurements of other physical activities (pedometer) were recorded, those weekly exercise minutes were set to the mean value of that individual.
Body mass index. Participants recorded their weight on a weekly basis, using the same diary that was used to report exercise activity (Supplemental Figure 1 in the online supplement). BMI was calculated using participants’ height that was self-reported upon enrollment with AlphaNet. For the purposes of this study, BMI categories were defined as follows: low BMI (BMI ≤19), normal BMI (BMI = 20–25), high BMI (BMI >25–30) and very high BMI (BMI >30).
Additional participant characteristics. AlphaNet collects demographic information upon enrollment with AlphaNet, which is updated on a monthly or quarterly basis. This information was used to describe the following characteristics of participants at the start of the SFS study: age, sex, oxygen use, race/ ethnicity, marital status, alpha-1 antitrypsin genotype, and smoking history (never smoker versus past/current smoker).
Statistical Analysis
SAS 9.4 was used to perform all statistical analyses (SAS Institute, Cary, North Carolina).For all analyses, significance tests were 2-sided with a significance level of 0.05.
Characteristics of participants. Descriptive statistics were computed for baseline characteristics of the participants and stratified by intervention arm. Continuous variables were reported as mean ± SD, and categorical variables as frequencies and proportions. To compare the values between the intervention arms, t-tests were used for continuous variables and chi-square tests for categorical variables. In addition, t-tests and chi-square tests were used to compare baseline characteristics of individuals who were included in analyses to individuals who were randomized but excluded from analyses due to lack of evaluable outcome data.
Exercise activity. All data were analyzed using intent-to-treat analysis. All individuals who provided at least 2 weeks of exercise activity data were included in the primary analysis regardless of how long they remained actively engaged with the study. Considering the complexity of longitudinal patient-reported outcomes of exercise activity data, the primary analyses of the effect of intervention on weekly exercise minutes were analyzed using a linear mixed model that incorporates both fixed and random effects.11 This methodology is robust to missing data as missing values at 1 measurement point do not prevent from including an individual’s data in the analyses. Based on the Akaike Information Criterion, unstructured covariance structure of the random effects was selected as the best fit to the data. The model included random effects of intercept and slope and fixed effect of intervention group (intervention versus standard care). The model also included an interaction term of group with time reflecting the effect of intervention over time. Sensitivity analyses for exercise activity were conducted without limiting maximum values of weekly exercise minutes or addressing zero exercise values. Missing data analyses compared baseline characteristics of the respondents included in the analyses and those excluded due to withdrawal or missing data.
Body mass index. BMI analyses also were based on intention-to-treat principles. All individuals who provided at least 2 weight measurements were included in the BMI analyses. The pre-intervention weight measurement was defined and calculated as an average of the first 2 weight measurements or a single first weight measurement if only 2 were recorded during the study period. The post-intervention weight measurement was computed the same way. BMI changes pre-post intervention were calculated and reported as a mean change and percentage change for each randomized group and stratified by baseline BMI categories. Pre-post BMI changes between intervention and standard groups were compared using a t-test. A Wilcoxon rank-sum test was used to compare pre-post BMI change between intervention and standard care groups stratified by baseline BMI categories. A signed rank test was used to evaluate significance of BMI change pre-post study within each intervention arm stratified by baseline BMI categories.
Results
Characteristics of Participants
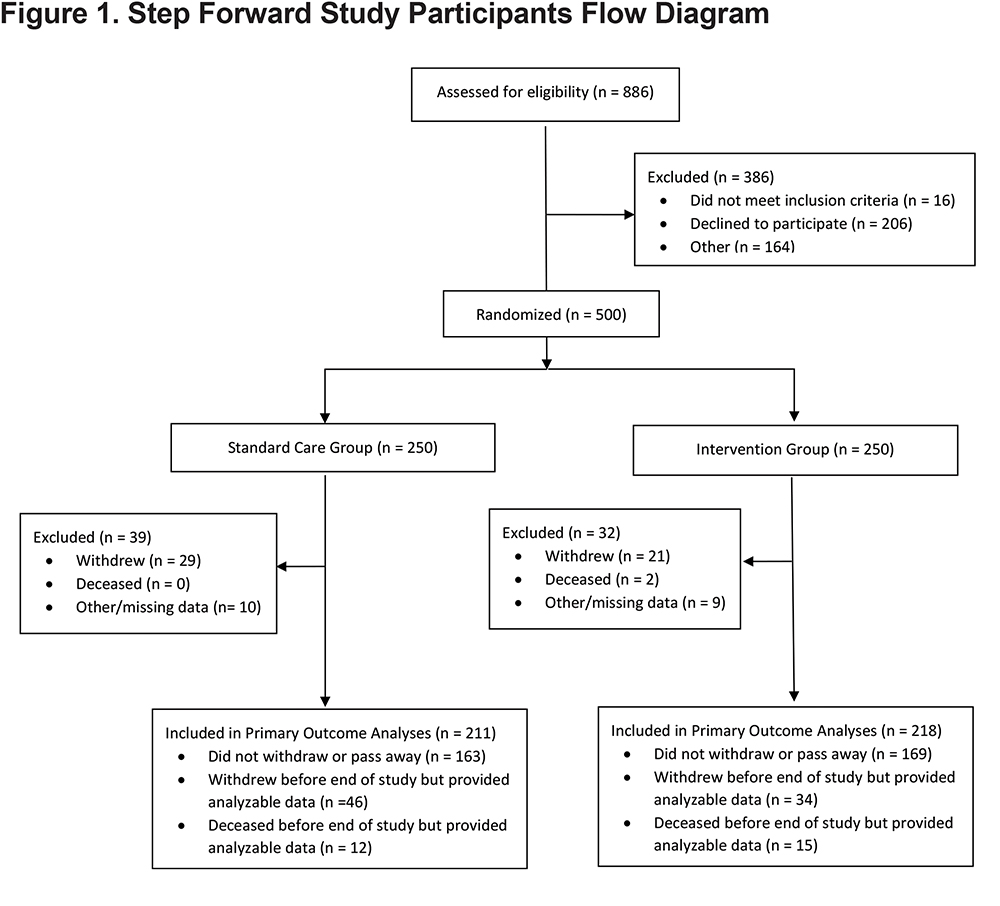
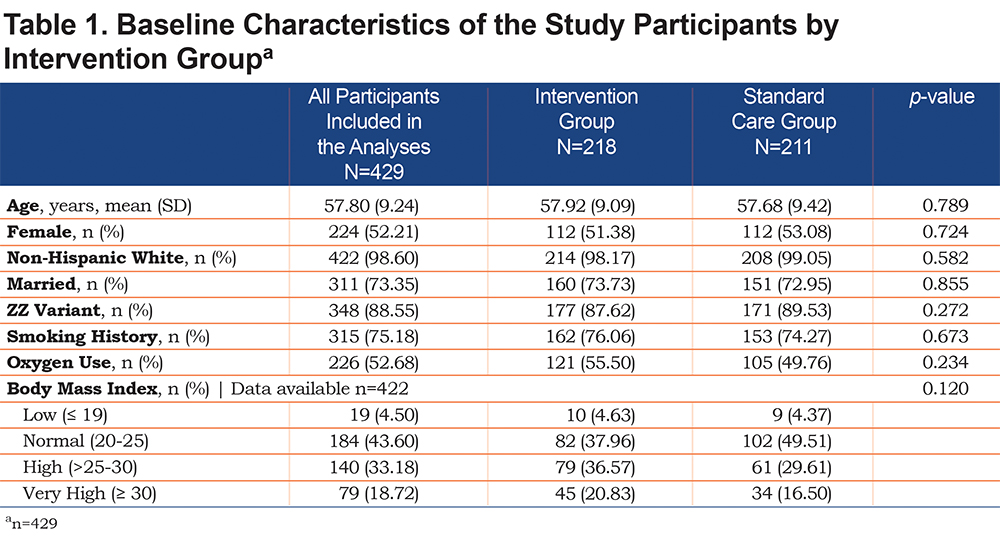
Figure 1 describes the flow of participants through the study. Of the 500 randomized participants, 429 provided at least 2 weeks of exercise activity data and therefore, were included in the analyses of the primary outcome. Table 1 shows the baseline characteristics of these 429 participants. Mean age of the participants was 57.8 years (SD=9.2), about half were female (52.2%), with predominantly ZZ genotype of AAT (88.6%). The majority of participants were non-Hispanic white (98.6%), married (73.4%), had a history of smoking (75.2%) and used oxygen (52.7%) at enrollment. At the start of SFS, approximately half of the participants had high or very high BMIs (51.9%). No significant differences were detected in baseline characteristics between the intervention arms (Table 1).
Exercise Activity
Among the 429 participants included in the primary analysis, 218 (50.8%) were randomized into the intervention group and 211 (49.2%) into the standard care only group. Each participant contributed on average 107.6 (SD=72.3) weeks of exercise activity data with no significant difference between the 2 groups.
Table 2 outlines the results of the linear mixed model. There was a significant effect of intervention on weekly exercise minutes over time as assessed by the significant interaction term of group-by-time (p=0.018 for difference in slopes between the 2 groups). The least square mean number of weekly exercise minutes in the intervention group was 167.14 minutes (95% CI: 146.21–188.07), compared to 148.31 minutes (95% CI: 126.84–169.78) in the standard care group. Participants in the intervention group had on average 18.83 (95% CI: -11.16–48.81) more minutes of weekly exercising compared to the standard care only group. Interestingly, at the start of the study, participants in the intervention group exercised on average 8.15 minutes less per week compared to the standard care group, although not a statistically significant difference (p=0.524 for difference in intercepts) (see parameter “Group” in Table 2).
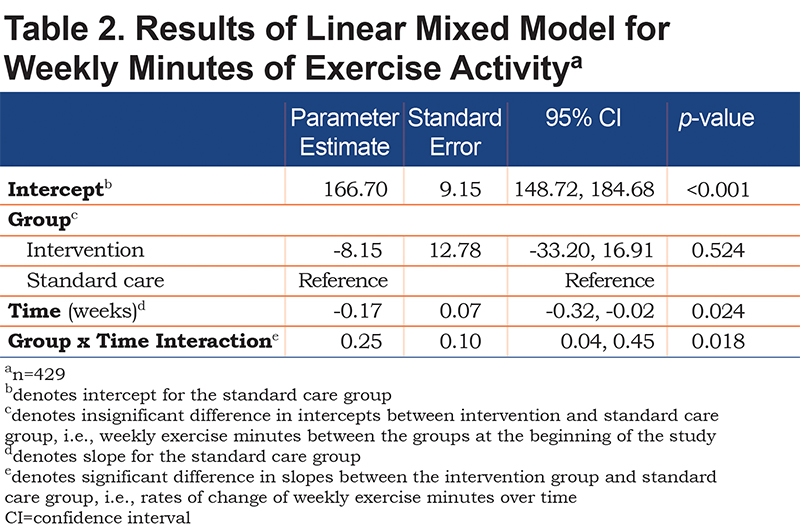
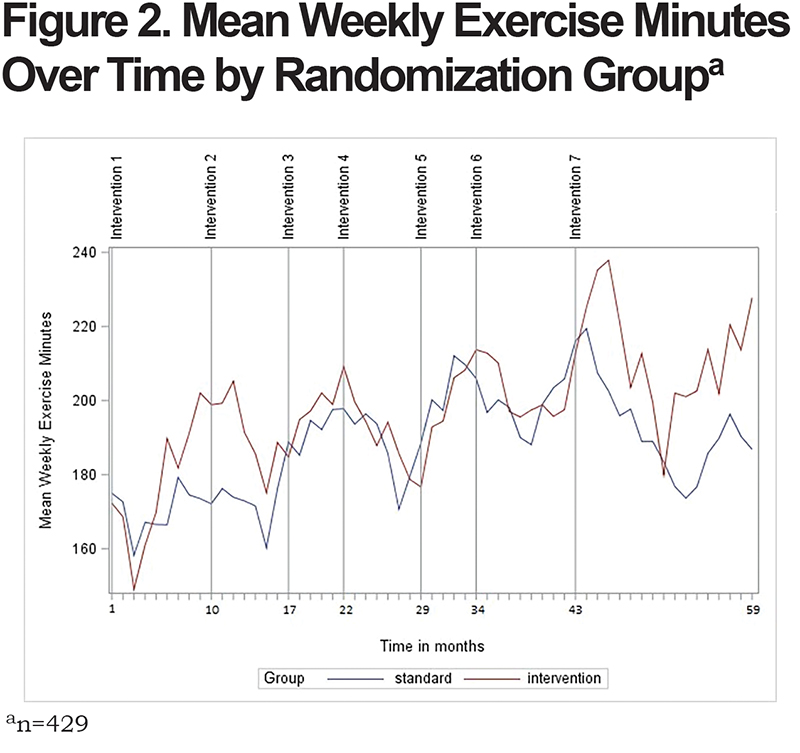
Figure 2 demonstrates the temporal effects of interventions on mean weekly exercise minutes by randomization arm. This was a multi-year study and participants experienced a change of seasons and holidays during the study. Plot in Figure 2 reflects the expected decline in a home-based study in exercising during winter months that are consistent across the randomization arms. These self-reported data demonstrate low peaks of exercising around months 3, 15, 27, 39, and 51 corresponding with the months of December of consecutive study years.
The sensitivity analyses examined the effect of limiting the high outliers of the exercise minutes and setting zero values of the exercise minutes to the mean per individual participant when other measurements of physical activity were present. Similar to the results of the primary analyses, the results of the linear mixed model in the sensitivity analyses showed greater number of exercise minutes in the intervention group (166.91 minutes [95% CI:141.69–192.12]) compared to the standard care group (146.32 minutes [95% CI: 120.45–172.19]). The intervention group reported an average of 20.59 more minutes of exercise activity per week (95%CI: -15.53–56.72). The interaction term between time and intervention group fell short of statistical significance (p=0.13).
Body Mass Index
A total of 422 participants were included in the BMI analyses: n=216 (51.18%) in the intervention group and n=206 (48.82%) in the standard care group. The 7 participants who were included in the exercise activity but not the BMI analyses did not significantly differ from the rest of the cohort in baseline characteristics. The pre-intervention BMI was calculated as the average of the first 2 weight measurements. These 2 weight measurements were 1 week apart for more than 90% of the participants who were included in the BMI analyses.
In the overall cohort, the pre-post study BMI decreased by 1.72% (mean decrease 0.51 [SD=1.93]). In the intervention group, BMI decreased by 2.56%, and in the standard care group BMI decreased by 0.84%. There was a significant difference in BMI change between the intervention group (mean BMI decrease 0.74, SD=2.16) and the standard care group (mean BMI decrease 0.27, SD=1.63); t(420)=-2.52, p=0.0122.
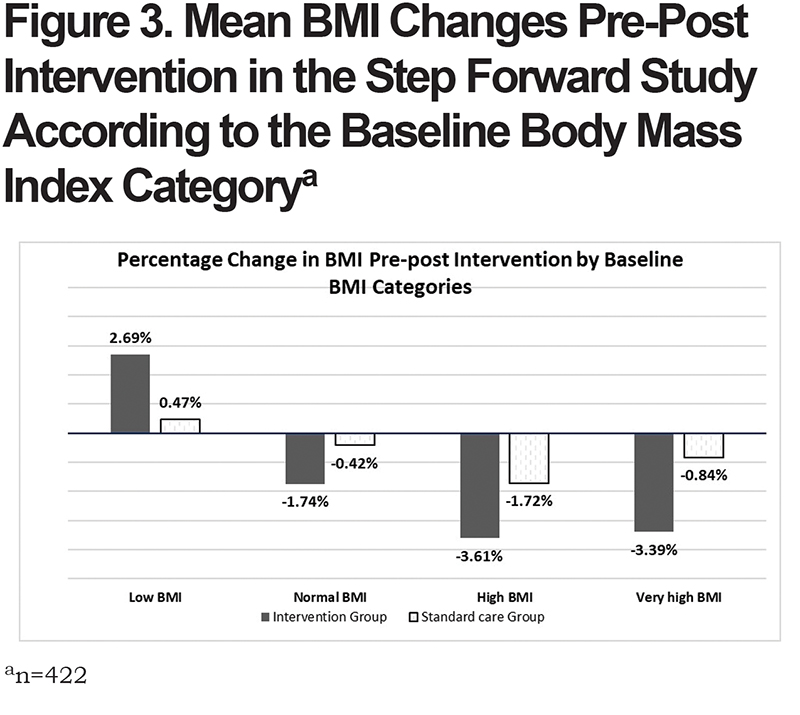
In addition, we performed stratified analyses of BMI change according to the baseline BMI category (Figure 3). Within the intervention arm, there was a significant BMI change pre-post study in the intervention group in the normal, high and very high BMI categories based on a signed rank test (p<0.01). Within the standard care arm, significant change in BMI pre-post study was identified in the high BMI category only (p=0.03). Comparing the BMI changes across the 2 randomization arms, individuals in the high and very high BMI groups had a greater BMI decrease in the intervention group (3.61% and 3.39% respectively) compared to the standard care group (1.72% and 0.84% respectively). Similarly, participants in the intervention group with low baseline BMI had a mean BMI increase of 2.69% compared to an increase only by 0.47% in the standard care group. Although, the Wilcoxon rank-sum tests for comparison in BMI change across the randomization arms did not reach statistical significance at alpha=0.05.
Missing Data Analyses
Individuals who were included in the primary analyses were compared to those who were excluded from analyses due to withdrawal or missing data (see Supplemental Table 3 in the online supplement). No significant differences were found between those included and excluded from the study in baseline characteristics (age, gender, AATD variant, baseline BMI) except for marital status. A greater proportion of study participants were married compared to those excluded (73.35% versus 60.30% married, respectively, p=0.03).
Discussion
In this interventional trial, we compared exercise activity and BMI of individuals who participated in an intensive distance intervention to those who participated in AlphaNet’s standard disease management program only.10 As hypothesized, an intensive intervention in which educational materials and guidance were tailored to each BMI category helped the participants to improve their exercise activity measured by weekly exercise minutes and move towards their ideal body weight. Interestingly, participants in the standard care group also reported relatively high average number of exercise minutes as well as improvements in their body weight. As described by Perkins et al, adherent participants in the ADMAPP program engage in regular exercising and perceive themselves being “pretty fit” and “in better health” compared to those who do not adhere to the recommendations of the management program.9 Regular mailings of SFS study materials to the standard care group may have increased adherence to ADMAPP and influenced improved exercise activities. Similarly, BMI improvements in the standard care group may be related to the fact that all participants in the study received general education materials and select fitness equipment tailored to their BMI category at enrollment. Study participation and these mailings may have had positive impacts and motivated the individuals to engage in exercising and weight management. Therefore, it is possible that the study underestimated the impact of the intensive intervention program.
The focus of the SFS study was underpinned by the knowledge that people with chronic lung illnesses often do not spend the recommended 30 min of daily exercising and do not develop and maintain advised levels of fitness.12 For the most part, symptom-induced inactivity has been shown to be one of the reasons for low exercise activities (deconditioning and muscle weakness).13 Although, exercise capacity in individuals with chronic lung diseases is reduced not only due to dyspnea but also due to skeletal muscle fatigue (mainly legs).14
In previous research, exercise capacity was determined to be one of the best predictors of health status (superior to pulmonary function tests and high-resolution computed tomography) in patients with AATD15,16 and other respiratory conditions such as COPD. Dowson et al suggested use of exercise capacity as an alternative outcome measure in intervention trials in patients with AATD.16 In addition, exercise capacity is one of the measures incorporated in the calculation of the Body mass index-airflow Obstruction-Dyspnea-Exercise capacity (BODE) index which is a prognostic mortality risk instrument for people with COPD.3,17 This highlights the importance of exercise capacity in improving health outcomes. Recent research also demonstrated association between poor 6-minute walk test and risk of mortality in COPD patients.18
Despite the benefits of the pulmonary rehabilitation programs in improving functional health status of patients with COPD and increasing their exercise capacity,19,20 the number of pulmonary rehabilitation facilities across the United States is insufficient.21 These centers often are located in urban areas and are not easily accessible by the patients living in rural areas who could benefit from these programs.
Many patients with AATD-related COPD develop a sedentary lifestyle that further reduces their exercise capacity. Therefore, it is important to quantify daily physical activities including minutes spent performing different types of activities,22 especially to assess fitness in people with chronic illnesses who tend to remain sedentary. Accountability by timing and recording length and types of fitness activities in daily diaries may have motivated participants in our study to continue exercising for the duration of the follow-up period.
Results of a recent survey of 518 patients with AATD demonstrated that the majority of patients with at least 1 deficient allele (68%) remain physically active and participate in purposeful exercising.15 And among patients receiving pulmonary rehabilitation, a majority report improvements in their quality of life.15 Pulmonary rehabilitation that includes physical exercising and patient education has been associated with improvement in respiratory symptoms and overall quality of life among patients with AATD,10 COPD and other chronic lung diseases.23
In healthy people, regular exercising has anti-inflammatory effects and health benefits.24 Earlier research raised concerns about increase in inflammatory burden caused by exercising in patients with COPD due to systemic inflammation.14 Although in their 2014 study, Olfert et al found no evidence to suggest an increase in acute or chronic inflammation in patients with AATD.25 Due to small sample size in their study, the researchers were not able to conclude whether aerobic exercise does indeed have a protective effect on disease progression (cause-and-effect relationship).
Patients with impaired lung function should maintain a healthy body weight and recommended daily exercise levels. Low BMI and malnutrition have been found to be associated with a diagnosis of emphysema, and high BMI was found to be prevalent in patients with chronic bronchitis.26 In a prospective study, low BMI was found to be a risk factor for development of COPD in males.27 In AATD and other COPD patients presenting with emphysema, low body weight is known to be associated with worse outcomes including greater morbidity and mortality,3,28 even controlling for pulmonary function,29 especially in advanced stages of the disease. In addition, the BMI decline in later stages of COPD correlates with a decrease in muscle mass and proportional drop in fat free mass index, which is more accurate in expressing variables of COPD severity such as the modified Medical Research Council scale and forced expiratory volume in 1 second.30
Similarly, high BMI also adversely affects outcomes in COPD patients. Risk of developing obesity in patients with COPD (and AATD) is mainly a result of reduced levels of exercise activities compared to healthy adults.12 In a study by Beiko et al, AATD patients with a BMI >30 were found to have greater exacerbation frequency.31 Similarly, in AATD-replete COPD, obesity is prevalent (35%) and is associated with worse patient-related outcomes, such as shorter 6-minute walk distance, higher St George’s Respiratory Questionnaire scores, and more frequent severe exacerbations.32
Strengths and Limitations
Several strengths of the study should be highlighted. To the authors’ knowledge, SFS is the first double-blind, randomized control study that examines effects of intensive distance intervention on health outcomes in patients with AATD. Other major strengths of the study include a long follow-up period of approximately 5 years of patient-reported health outcomes and use of a variety of tailored distance intervention methods including mailings, teleconferences, and VPR. Exercise activity data were collected daily throughout the 5-year study period which provides remarkable granularity compared to other studies that evaluate exercise activity at several discrete time points. In addition, utilizing a unique network of AlphaNet coordinators who stayed in close contact with a geographically diverse study population throughout the study period presents a major strength of the SFS.
Results of the study should be interpreted in light of limitations. Both outcome measures were self-reported and hence, measurement error is a possibility. This may have resulted in an inaccurate estimate of the true effect of the intervention. Social desirability bias is often present in studies of physical exercise based on self-reporting33 and may have led to over-reporting of exercise minutes. If present, the non-differential bias between both study arms would attenuate the effect of the intensive intervention. In addition, selection bias cannot be excluded considering the especially motivated cohort of participants compared to a general population of patients with COPD. The study was designed with the goal of blinding participants with regard to treatment allocation. To achieve this goal, participants in the standard care group received some of the study-related materials (e.g., pedometers), and therefore, all participants in this study received some additional intervention beyond AlphaNet’s standard disease management program. An additional limitation is that the AlphaNet coordinators who interacted regularly with participants throughout the study were not blinded with regard to treatment assignment, and participants may have discussed interventions with each other and, therefore, have determined which treatment group they were in. Thus, it is possible that some participants did not remain blind with regard to their treatment assignment throughout the study. The fact that there was no differential in the drop-out rates between the standard care and intervention groups argues against a significant effect of any loss of blinding among participants. An additional limitation is related to a potential for a non-response bias mainly due to missing data from patients not recording all scheduled daily and weekly dairy measurements. Missing data is a common occurrence in prospective studies with a long follow-up period. Our missing data analyses did not find significant differences in main baseline characteristics between those included in the study and excluded due to incomplete data.
Conclusion
In summary, adding an intensive fitness intervention to standard ADMAPP care resulted in increased exercise activities and improvements in BMI among individuals with AATD-associated lung disease.
Acknowledgements
Author contributions: RC provided data analysis and interpretation of the results, wrote the original draft and was involved in the editing and revisions of the manuscript prior to submission. DMM, KEH, and RAS were involved in the interpretation of the results and had substantial involvement in the manuscript's revisions and editing. BB and RAS were responsible for the conception and design of the study and RAS was responsible for acquisition of the data. TB had substantial involvement in the manuscript's revisions and editing.
This work was funded by AlphaNet, Inc., United States. We are extremely grateful for the superb work of all the AlphaNet Coordinators. We also thank the AlphaNet subscribers who participated in this study. Much of the planning and execution of this study was done by the late Bonnie Boyd, BSN, AlphaNet’s Director of Disease Management and Research. Videos and additional materials used in this study’s Virtual Pulmonary Rehab program were prepared for AlphaNet, Inc., by Sherrie K. Evenson, MS, clinical exercise physiologist and Joann M. Bonneville, RN, BSN, pulmonary rehab nurse.
Declaration of Interest
DMM is a former employee and current shareholder of GlaxoSmithKline. RAS has served on advisory boards and/or as a speaker for Grifols and CSL Behring. He is on the board of directors of AlphaNet and serves as the Medical Director of AlphaNet. KEH has received consulting income from AlphaNet. RC has received research support from AlphaNet. TB reports no conflicts.