Running Head: Emphysema and fSAD Progression in Smokers
Funding support: The project described was supported by grants R01HL089897 and R01HL089856 from the National Heart, Lung, and Blood Institute. The COPD Genetic Epidemiology (COPDGene®) study is also supported by the COPD Foundation through contributions made to an industry advisory board representing AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline. In addition, this project has been financially supported by the Foundation “De Drie Lichten” in the Netherlands, the Lung Foundation Netherlands (grant 9.1.15.067FE), and the Foundation “Stichting Astma Bestrijding.”
Date of acceptance: November 19, 2020 | Published online: December 2, 2020
Abbreviations: forced expiratory volume in 1 second, FEV1;chronic obstructive pulmonary disease, COPD; computed tomography, CT; functional small airways disease, fSAD; Global initiative for chronic Obstructive Lung Disease, GOLD; odds ratio, OR; preserved ratio- impaired spirometry, PRISm; St George’s Respiratory Questionnaire, SGRQ; 6-minute walking distance, 6MWD; modified Medical Research Council dyspnea score, mMRC; total lung capacity, TLC; Hounsfield unit, HU; functional residual capacity, FRC
Citation: Pompe E, Moore CM, Mohamed Hoesein FAA, et al for the COPDGene Investigators. Progression of emphysema and small airways disease in cigarette smokers. Chronic Obstr Pulm Dis. 2021; 8(2): 198-212. doi: http://doi.org/10.15326/jcopdf.2020.0140
Online Supplemental Material: Read Online Supplemental Material (557KB)
Introduction
Cigarette smoking remains common around the world and is responsible for a wide range of diseases including chronic obstructive pulmonary disease (COPD). After 25 years of smoking, only a minority of individuals will develop COPD by spirometric criteria,1 but the spirometric diagnosis of COPD likely underestimates the burden of this smoking-related lung disease. Indeed, it has been shown that more than half of current and former smokers who do not meet the spirometric criteria for COPD have emphysema and may have significant respiratory symptoms.2,3
Smoking cessation alone does not eliminate lung disease, and smoking-related abnormalities may still progress after smoking cessation. Identification of individuals at risk for progression is, therefore, of great importance since early intervention and treatment may prevent clinical deterioration. Computed tomography (CT) quantification techniques could be valuable in the identification of the presence and progression of smoking-related changes in the airways and the lungs, as they can provide information that would amplify and augment spirometric data.4-6
The associations between changes in forced expiratory volume in 1 second (FEV1) and baseline CT-derived emphysema and functional small airway disease (fSAD) have been evaluated in individuals with early disease and showed that fSAD at baseline is associated with FEV1 decline over time.6,7 This could mean that airway disease precedes lung function decline and is in keeping with the hypothesis that airway loss and obstruction are precursors of emphysema. Indeed, in a more recent study, it has been shown that fSAD seems to be a radiologic precursor of progression in emphysema.7 However, how this relates to changes in lung function remains unknown. Also, results on the relationship between other clinical parameters such as the St George’s Respiratory Questionnaire (SGRQ) and the 6-minute walking distance (6MWD) and emphysema progression are lacking. Yet, it is important to determine how quantitative CT parameters of emphysema and fSAD progress over time and to assess their relationship to changes in clinical parameters and FEV1.
The main objective of this study was to determine factors that contribute to progression of emphysema and FEV1. To accomplish this, 5-year follow-up data from the large multi-center COPD Genetic Epidemiology (COPDGene©) study was used and baseline clinical and CT imaging variables were evaluated for their association with emphysema and FEV1. A primary focus was to identify factors associated with disease progression in individuals with little or no airflow obstruction at baseline.
Methods
Participants
Participants for this study were participants in the COPDGene study.8 This multicenter observational cohort study included current and former smokers with or without airflow obstruction and a smoking history of ≥10 pack years. In addition, healthy nonsmokers with a lifetime smoking history of <100 cigarettes, no history of lung disease, and normal post-bronchodilator spirometry were recruited.
In the present study, we examined the first 5000 COPDGene participants who returned for 5-year follow-up visits. A total of 40 participants were nonsmokers. An additional 32 nonsmokers who returned for their follow-up were added to increase the amount of nonsmoking data. The total number of participants was reduced to 3088 after exclusion of participants because of several reasons as shown in the consort diagram (Figure S1 in the online supplement). Two nonsmoking participants were excluded as scanner calibrations differed from the rest of the nonsmokers. Longitudinal spirometry data was missing in 44 participants, but data on emphysema progression was present. The COPDGene study was approved by the institutional review board at each center, and all participants provided written informed consent.
Pulmonary Function Tests
All participants underwent pre- and post-bronchodilator spirometry. Post-bronchodilator spirometry was performed (ndd Easy-One spirometer, Andover, Massachusetts) and airflow obstruction (i.e., diagnosis of COPD) was defined by a post-bronchodilator FEV1/FVC<0.70 according to the Global initiative for chronic Obstructive Lung Disease (GOLD) guidelines and disease severity was defined by GOLD stage.9 Participants with FEV1/FVC≥0.70, but with FEV1% predicted <80% were considered to have preserved ratio-impaired spirometry (PRISm).10
Clinical Parameters
Data on smoking history was recorded at baseline and follow-up by using standard questionnaires.8 Additionally, respiratory quality of life was assessed using the SGRQ.11 The modified Medical Research Council (mMRC) dyspnea score,12 and 6MWD were also assessed.
Computed Tomography-Acquisition and Micro-mapping
All participants underwent paired inspiratory and expiratory CT scans at maximal inspiration (total lung capacity [TLC]) and end-expiration using a standardized protocol.8 The lungs were segmented automatically using Thirona Lung Quantification software (Nijmegen, the Netherlands) and segmentations were visually checked.13 The micro-mapping technique was applied using a technique similar to parametric response mapping described by Galbán et al.14,15 In brief, the inspiratory and expiratory CT scans were spatially co-registered, after which, voxels were classified based on their inspiratory and expiratory Hounsfield unit (HU).14 All voxels <-950HU on inspiratory CT and <-856HU on expiratory CT were classified as emphysematous; voxels between -950HU and -500HU on inspiratory CT and <-856HU on expiratory CT were classified as fSAD; voxels between -950HU and -500HU on inspiratory CT and between -856 and -500HU on expiratory CT were classified as normal lung tissue. Voxels below -950HU on inspiratory CT, but above -856HU in the expiratory CT were classified as “other.” The number of voxels per category were expressed as a percentage of all classified voxels to achieve relative lung volumes.
Statistical Analysis
A linear mixed model was created for log (emphysema) as a function of age, gender, race, body mass index (BMI), smoking status, pack years, functional residual capacity (FRC)/TLC derived from CT, and observation time. Additionally, the interaction between observation time and clinical variables was included to evaluate differences between participants in progression over time. Random intercepts were included for each participant, study center, and scanner make/model to account for correlation due to repeated measurements on participants over time, clustering of participants in study centers, and the effect of different CT scanner models. Similar models were created for log (FEV1) (not including FRC/TLC derived from CT and the effect of different CT scanner models).
Logistic regression models were used to test associations between baseline characteristics and the odds of emphysema progression. Participants were defined as “progressing” if their emphysema and/or FEV1 changed beyond the 95th percentile of change observed in nonsmokers (0.25% emphysema progression per year; 89 mL decrease in FEV1 per year). Similarly, the presence of baseline emphysema was defined as having more than the 95th percentile in nonsmokers (>1.31%). Generalized estimating equations were used to account for clustering of participants in study centers. A baseline model was created using clinical characteristics (age, gender, race, BMI, smoking status, and pack years) after which the presence of baseline emphysema, the amount of fSAD, 6MWD, SGRQ, and the presence of self-reported exacerbations at baseline were added to the model separately. An additional model was created including an interaction between fSAD and the presence of baseline emphysema to evaluate the association of fSAD and emphysema progression in the absence of baseline emphysema. Analyses were stratified by GOLD stage. A similar set of models was fit using FEV1 progression as the outcome. Sensitivity analyses were performed using cut-off values at the 90th and 97.5th percentile (cut-off values of 0.14% and 0.45% emphysema per year, and 76 mL and 100 mL in FEV1 per year, respectively). In addition, since a change of 76 mL in FEV1 per year is still quite high, we performed 2 additional sensitivity analyses. Multivariate logistic regression models for FEV1 were created where FEV1 progression (yes/no) was defined as a decline in FEV1 of greater than 2% or 4% per year, resulting in declines of approximately 10% or 20% from baseline over the typical 5-year follow-up.
The percentage of voxels that shifted from one category to another, at follow-up as compared to baseline, were evaluated separately for participants who progressed and participants who did not progress. P-values of <0.05 were considered statistically significant. As presented, p-values are not adjusted for multiple comparisons (more than 40 statistical tests were performed). Analyses were performed using SPSS 23.0 (Chicago, Illinois) and R 3.2.3 (the R Foundation, Vienna, Austria).
Results
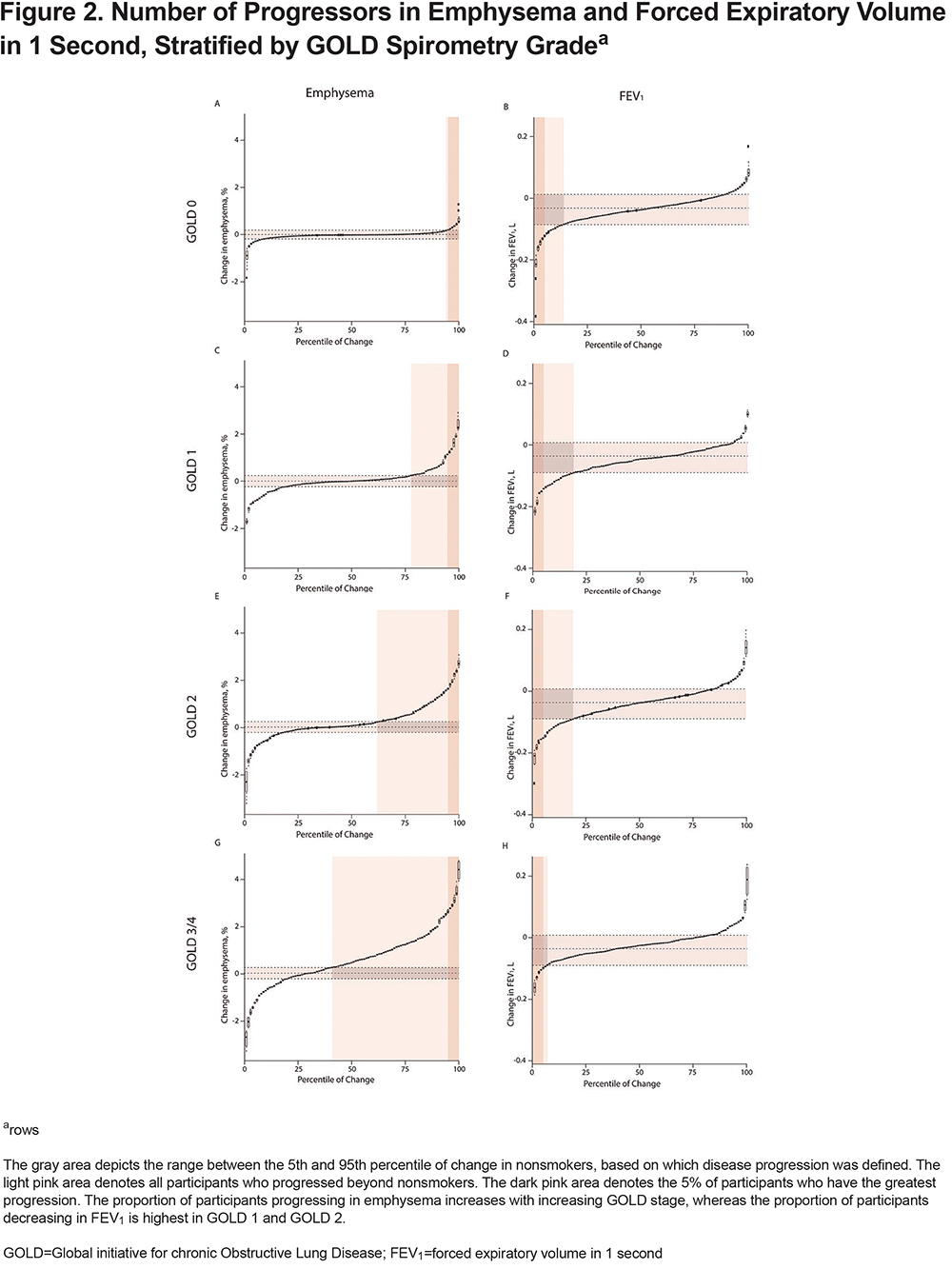
Of 3088 included participants, 72 (2.3%) participants were nonsmokers, 1430 (46.3%) were classified as GOLD 0, 305 (9.9%) as PRISm, 267 (8.6%) as GOLD 1, 616 (19.9%) as GOLD 2, 332 (10.8%) as GOLD 3, and 66 (2.1%) as GOLD 4, based on Phase-1 spirometry. In further analyses, GOLD 3 and GOLD 4 are grouped together (total 398 participants). Mean±SD follow-up time was 5.3±0.5 years for current/former smokers and 5.5±0.8 years for nonsmokers. Baseline characteristics are shown in Table 1. Mean±SD change in FEV1, emphysema-like voxels, and fSAD per year in nonsmokers was -31±29 ml, 0.003±0.13%, and 0.08±0.76%, respectively. Overall change in emphysema, fSAD, and FEV1 beyond nonsmokers is shown in Figure 1. Figure 2 is stratified by GOLD stage and shows results for emphysema and FEV1. Change in fSAD is shown in Figure S2 in the online supplement and results for the PRISm group are shown in Figure S3 in the online supplement. Baseline characteristics stratified by progression group are shown in Table S1 in the online supplement.
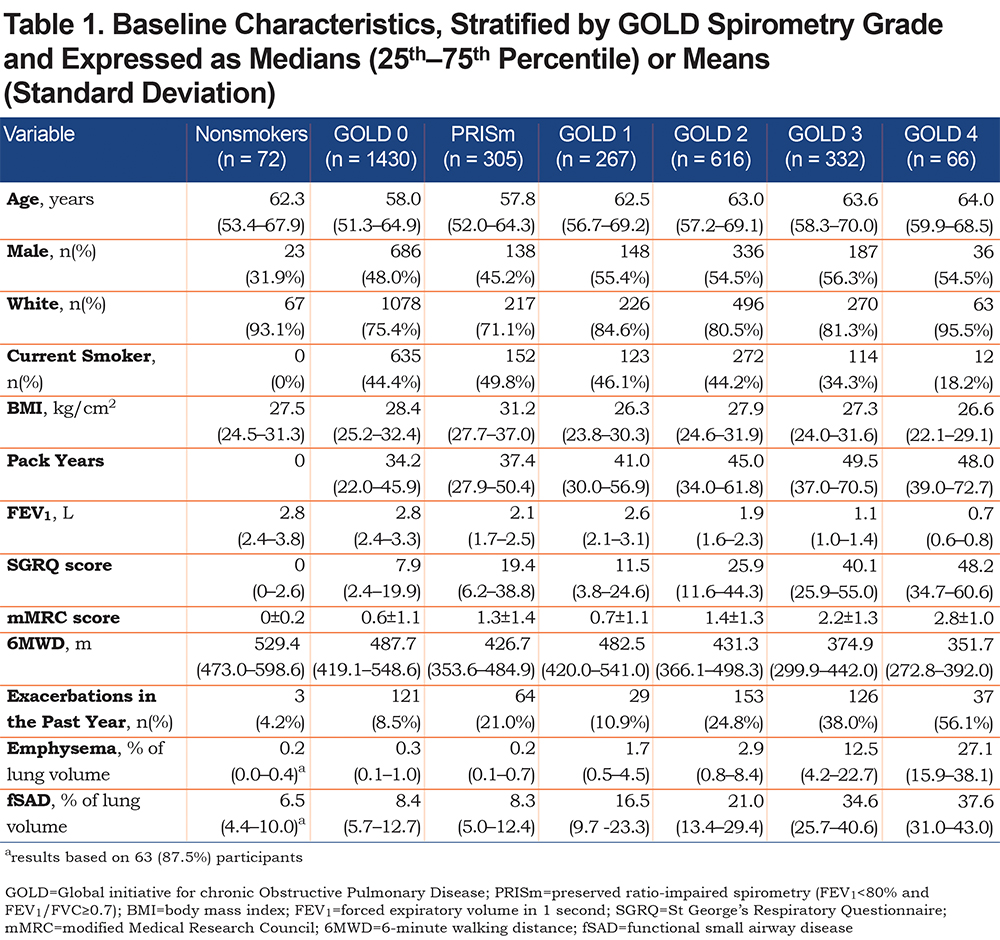
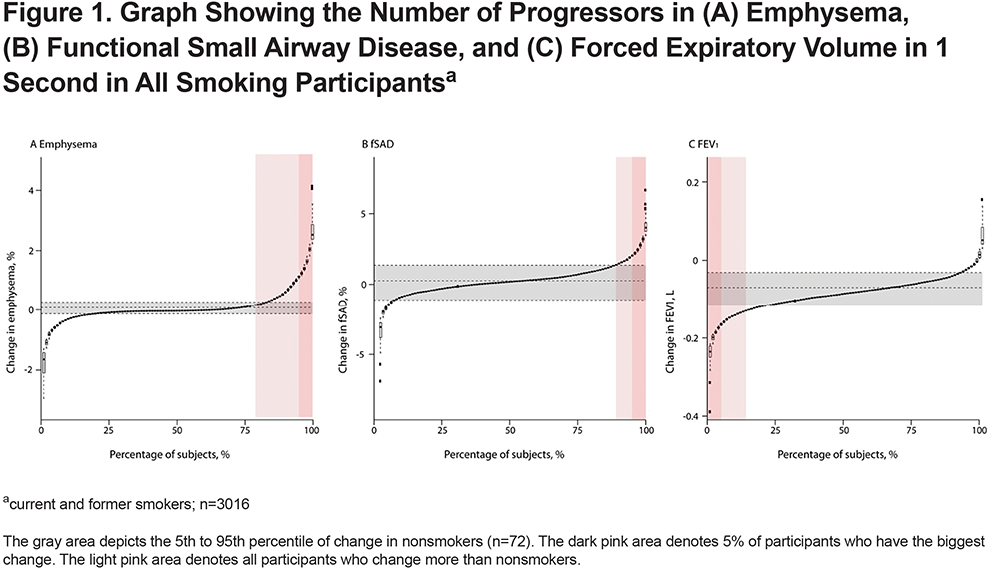
GOLD 0
In GOLD 0 participants, the median (25th–7th percentile) percentage of emphysema at baseline was 0.3% (0.1–1.0). Median (25th–75th percentile) change in emphysema per year was 0.0% (-0.05–0.03). Complete results of longitudinal multivariate analyses with emphysema and FEV1 as outcome are shown in Table S2 and Table S3 in the online supplement. Male gender and lower BMI were associated with larger increases in emphysema (p=0.004 and p<0.001, respectively).
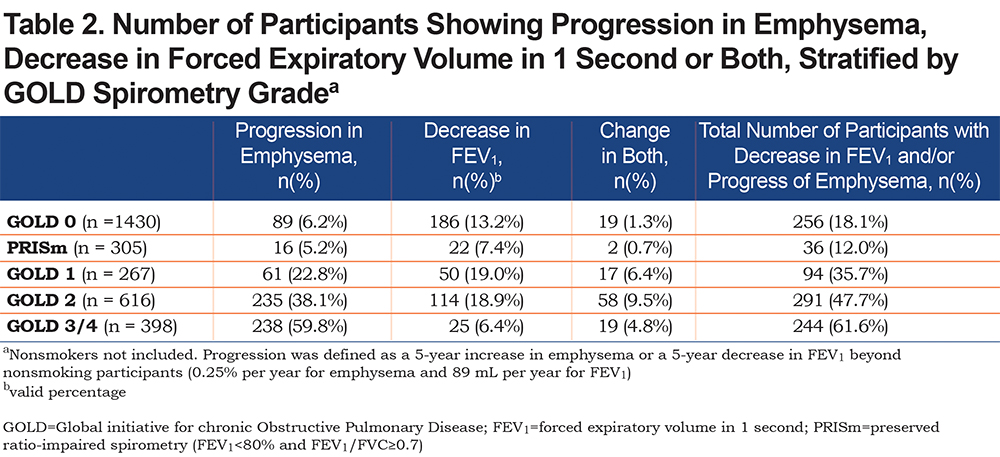
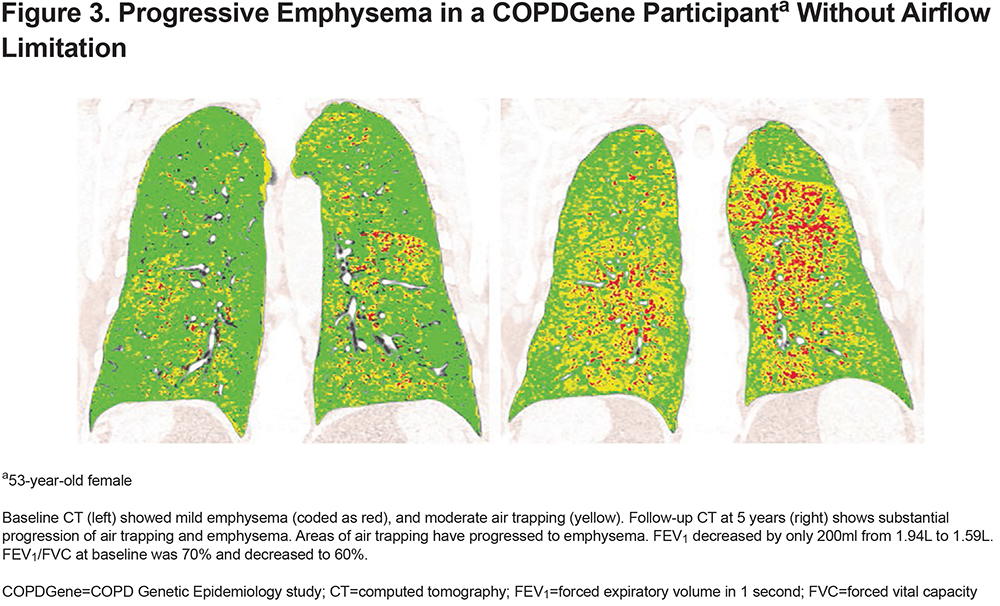
Progression in emphysema (compared with nonsmokers) occurred in 89/1430 (6.2%) participants; decrease in FEV1 occurred in 186 (13.0%); and progression in emphysema and decrease in FEV1 occurred in 19 (1.3%) participants. The number of participants who progressed is shown in Table 2. An example of a CT-scan of a GOLD 0 participant progressing in emphysema is shown in Figure 3.
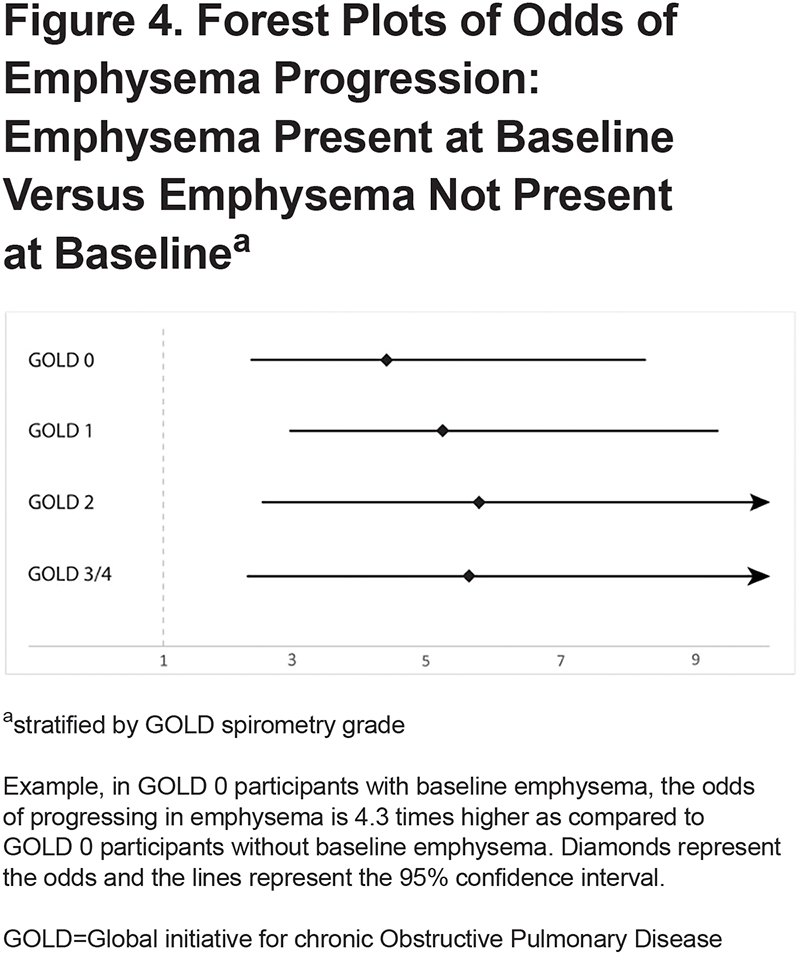
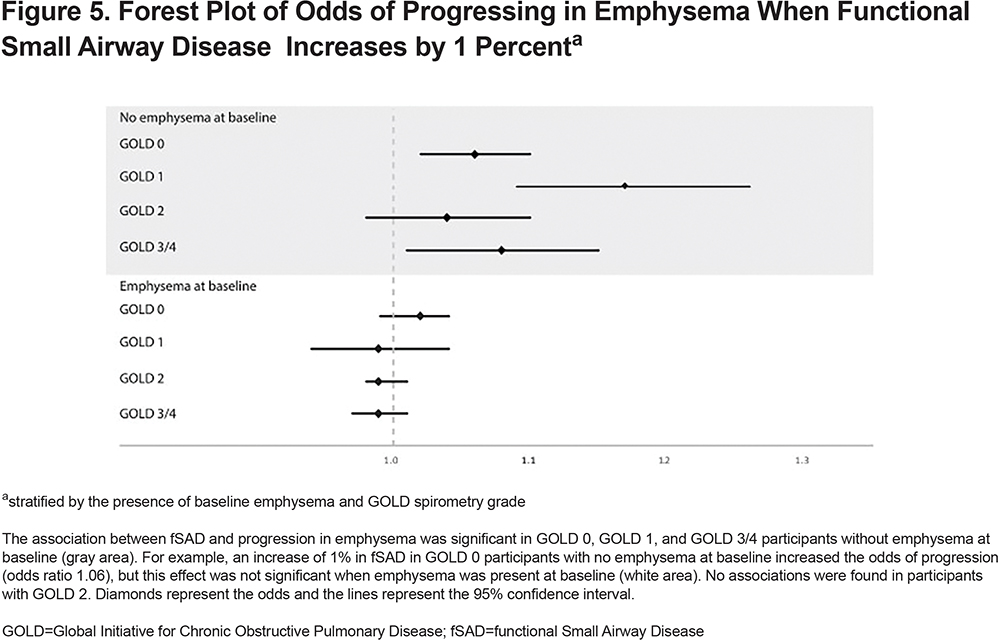
Generalized estimating equation models showed that the presence of emphysema at baseline increased the odds of progressing in emphysema (odds ratio [OR]:4.32, 95% CI 2.30 to 8.14) (Figure 4). Baseline fSAD was associated with emphysema progression as well (OR for 1% increase: 1.06, 95% CI 1.04 to 1.09). fSAD did not associate with emphysema progression for participants with emphysema at baseline (OR for 1% increase: 1.02, 95% CI 0.99 to 1.04). However, in the absence of baseline emphysema, fSAD did associate with emphysema progression (OR for 1% increase: 1.04, 95% CI 1.00 to 1.09) (Figure 5). Other clinical predictors of emphysema progression were lower 6MWD (OR for 100m increase: 0.99, 95% CI 0.99 to 0.99), higher SGRQ (OR for 1-unit increase: 1.02, 95% CI 1.00 to 1.03), higher mMRC (OR for 1-unit increase: 1.26, 95%CI 1.01 to 1.56), and the presence of exacerbations in the past year (OR:1.98, 95%CI 1.32 to 2.96). The only baseline predictor of a decrease in FEV1 was SGRQ (OR for 1-unit increase: 1.01, 95%CI 1.01 to 1.02). Complete results are shown in Table S4 and Table S5 in the online supplement.
PRISm
In PRISm participants, the median (25th–75th percentile) percentage of emphysema at baseline was 0.2% (0.1–0.7). Median (25th–75th percentile) change in emphysema per year was 0.0% (-0.02–0.04). Higher age and white race were associated with a higher increase in emphysema over time (p=0.04 and p=0.04, respectively).
Progression in emphysema occurred in 16/1430 (5.2%) participants; decrease in FEV1 occurred in 22 (7.2%); progression in emphysema and FEV1 occurred in 2 (0.7%). The number of emphysema progressors and participants decreasing in FEV1 did not allow for multivariate comparisons. In the sensitivity analyses where a cut-off at the 90th percentile was used, multivariate analyses were possible with 40 (13.1%) participants progressing in emphysema. This model showed that the presence of emphysema at baseline was associated with emphysema progression (OR:5.73, 95% CI 1.98 to 16.61). fSAD at baseline was significantly associated with emphysema progression as well (OR:1.11, 95% CI 1.05 to 1.18). For participants with emphysema at baseline, fSAD was not associated with emphysema progression (OR for 1% increase: 0.98, 95% CI 0.88 to 1.10). In the absence of baseline emphysema, fSAD did associate with emphysema progression (OR for 1% increase: 1.20, 95% CI 1.05 to 1.38). Another clinical predictor of emphysema progression in PRISm participants was the presence of exacerbations in the past year (OR:1.75, 95% CI 1.06 to 2.87).
GOLD 1
In GOLD 1 participants, the median (25th–75th percentile) percentage of emphysema at baseline was 1.7% (0.5–4.5). Median (25th–75th percentile) change in emphysema per year was 0.02% (-1.13–0.21). Changes in emphysema over time did not significantly differ by the demographic factors included in the model.
Progression in emphysema (compared with nonsmokers) occurred in 61/1430 (22.8%) participants; decrease in FEV1 occurred in 50 (18.7%); progression in emphysema and decrease in FEV1 occurred in 17 (6.4%) participants. The presence of emphysema at baseline associated with emphysema progression (OR:5.16, 95% CI 2.89 to 9.22). fSAD at baseline associated with emphysema progression as well (OR:1.06, 95% CI 1.03 to 1.10). Similar to GOLD 0, for participants with emphysema at baseline, fSAD was not significantly associated with emphysema progression (OR for 1% increase: 0.99, 95% CI 0.94 to 1.04), but in the absence of baseline emphysema, fSAD did associate with emphysema progression (OR for 1% increase: 1.16, 95% CI 1.07 to 1.25). Other clinical predictors of emphysema progression were lower baseline 6MWD (OR for 100m increase: 0.99, 95% CI 0.99 to 0.99) and higher baseline SGRQ (OR for 1-unit increase: 1.03, 95% CI 1.01 to 1.05). Baseline predictors of a decrease in FEV1 were fSAD at baseline (OR for 1% increase: 1.05, 95% CI 1.01 to 1.09), the presence of emphysema (OR: 3.32, 95% CI 1.49 to 4.59), and an increase in mMRC (OR for 1-unit increase: 1.35, 95% CI 1.11 to 1.64).
GOLD 2
In GOLD 2 participants, the median (25th–75th percentile) percentage of emphysema at baseline was 2.9% (0.8 – 8.4). Median (25th–75th percentile) change in emphysema per year was 0.08% (-0.08–0.54). Higher age and lower BMI were associated with a higher increase in emphysema over time (p=0.02 and p<0.001, respectively).
Progression in emphysema occurred in 235/1430 (38.1%) participants and decrease in FEV1 occurred in 114 (18.5%); progression in emphysema and decrease in FEV1 occurred in 58 (9.4%) participants. The presence of emphysema at baseline was associated with higher odds of emphysema progression (OR: 5.69, 95% CI 2.48 to 13.06). Baseline fSAD was associated with emphysema progression as well (OR for 1% increase in fSAD: 1.04, 95% CI: 1.01 to 1.06). Within the subgroup of participants with emphysema at baseline, fSAD was not associated with emphysema progression. Other clinical parameters were not associated with emphysema progression. Baseline predictors of a decrease in FEV1 were baseline fSAD (OR for 1% increase: 1.07, 95% CI 1.05 to 1.08), baseline fSAD in the presence of emphysema (OR for 1% increase: 1.06, 95% CI 1.04 to 1.08), and the presence of emphysema alone at baseline (OR:4.75, 95% CI 2.87 to7.83).
GOLD 3/4
In GOLD 3/4 participants, the median (25th–75th percentile) amount of emphysema at baseline was 14.9% (5.7–25.6). Median (25th–75th percentile) change in emphysema per year was 0.5% (-0.1–1.2). Lower BMI and more pack years were associated with a higher increase in emphysema over time (p<0.001 and p=0.02, respectively).
Progression in emphysema occurred in 238/1430 (59.8%) participants; decrease in FEV1 occurred in 25 (6.3%); progression in emphysema and decrease in FEV1 occurred in 19 (4.8%) participants. The presence of baseline emphysema was significantly associated with emphysema progression (OR: 5.55, 95%CI 2.26–13.58). fSAD at baseline was not associated with emphysema, but in the absence of baseline emphysema fSAD did associate with emphysema progression (OR for 1% increase: 1.08, 95%CI 1.01–1.15). The presence of exacerbations was associated with emphysema progression as well (OR: 1.52, 95%CI 1.09–2.11). The number of participants decreasing in FEV1 did not allow for multivariate comparisons.
Sensitivity Analysis
Sensitivity analyses using cut-off values at the 90th and 97.5th percentile resulted in cut-off values of 0.14% and 0.45% emphysema per year, and 76 mL and 100 mL in FEV1 per year, respectively. In the model using the 90th percentile of change in emphysema some but not many associations were lost such as the association with mMRC in GOLD 0, the association with fSAD in GOLD 1, the association with fSAD without baseline emphysema in GOLD 3/4, and the amount of exacerbations in GOLD 3/4 (Table S6). In the analysis using the 97.5th percentile of change many associations with emphysema progression were similar, but the association with fSAD without baseline emphysema was lost in GOLD 0 and GOLD 3/4 (Table S7). For GOLD 0, this may be due to the reduced sample size of participants defined as progressing using the stricter 97.5th percentile cutoff, as the magnitude of the association between emphysema progression and fSAD without baseline emphysema was similar.
In the model using the 90th percentile of change in FEV1 associations were found between fSAD, emphysema, and FEV1 in GOLD 0 (Table S8). Using the 97.5th percentile of change in FEV1 resulted in losing the association with fSAD without emphysema in GOLD 2, but all other associations were similar to the 95th percentile of change (Table S9).
In the model using FEV1 decline of 10% from baseline (2% decrease in FEV1 per year), associations were found between fSAD, emphysema, 6MWD, and SGRQ and FEV1 progression in GOLD 0 and GOLD 2 that were not present in the 95th percentile model, but results were similar to the 90th percentile of change threshold of 76 mL per year. Using FEV1 decline of 20% to define progression, resulted in losing these associations. Complete results are shown in Supplementary Table S10 and S11. A list of robust parameters, significant in all sensitivity analyses, is shown in Table S12.
Voxel Shifting
The percentages of voxels that shift from one category to another are shown in Table S13 to Table S17. Results of these tables show that in GOLD 0 participants who progress in emphysema, 16.6% of normal voxels shift to fSAD voxels and 9.8% of fSAD voxels shift to emphysema voxels, compared to 5.6% and 0.2% in GOLD 0 participants that did not progress (both p<0.001). The same trend is seen in PRISm participants, GOLD 1 participants, and GOLD 3/4 participants.
Discussion
This study demonstrates that the presence of emphysema at baseline is strongly associated with emphysema progression, even when there is no airflow limitation at baseline. In addition, we found that fSAD was associated with emphysema progression, even in participants without baseline emphysema. Further, we showed that change in emphysema is not always accompanied by significant changes in FEV1 and may, therefore, represent relatively independent processes.
The most important finding of our study is that the presence of emphysema is associated with emphysema progression in all GOLD stages, even in individuals without COPD. This means that even at an early stage when there is no demonstrable spirometric deterioration, evaluating the presence of emphysema can identify individuals at risk of progression. Disease progression in GOLD 0 participants was mainly characterized by an increase in emphysema rather than spirometric deterioration, suggesting a limited role of spirometry in early disease.
A second relevant finding of this study is that in early disease (GOLD 0, PRISm, and GOLD 1), fSAD at baseline predicted the development of emphysema when no emphysema was present. This is in line with the hypothesis that small airways disease precedes emphysema and is consistent with the findings by Hogg et al using micro CT and magnetic resonance imaging.16 Indeed, when evaluating voxel-to-voxel shift, we found that in GOLD 0 participants progressing in emphysema, 9.8% of fSAD voxels shifted to emphysema versus 1.7% in participants who did not progress. The same trend was seen in GOLD 1 and PRISm. In addition, 16.6% of previously normal voxels progressed to fSAD in GOLD 0 participants who progressed in emphysema, suggesting that progressive emphysema is accompanied by progression of fSAD. A similar analysis using this voxel-to-voxel shift was performed by Labaki et al7 in a subset of the COPDGene cohort where participants were only included when scanned on the same scanner make and model, and if there was <15% difference in inspiratory lung volumes between baseline and follow-up (total n=725). They found that disease progression was mainly characterized by transition of normal or fSAD voxels to emphysema and showed that fSAD and emphysema are independent predictors of emphysema development.7 It is, however, important to realize that this represents a complex technique in which reproducibility is limited. Indeed, in this study, voxels being defined as “abnormal” (mainly fSAD) also shifted back to normal lung density. Nevertheless, the results of both studies are important for further research in the development of this disease with which early identification and preventative therapy could limit disease progression.
This study showed that in early disease, change in FEV1 and emphysema overlapped only minimally and appear to be addressing different manifestations of disease in the early stages. Indeed, in a recent study performed by our group,17 we showed that progression in emphysema could only partly be explained by changes in FEV1. Airway disease has been shown to contribute substantially to airflow obstruction,18 and FEV1 deterioration is likely more pronounced when significant airway inflammation and/or destruction is present. This is in line with our finding of the predictive value of fSAD for lung function decline in GOLD 1 and GOLD 2 participants. Altogether, the results suggest that spirometry and imaging complement each other and can identify different elements of progression in chronic smoking-related lung disease.
We based our thresholds to identify both the presence and progression of emphysema on a group of non-smokers studied in the COPDGene cohort. Although this group was fairly small, it is the largest group of non-smokers with 5-year CT follow-up available. The threshold for the presence of baseline emphysema (>1.31%) as defined with CT was comparable to earlier results from the NELSON trial, who found thresholds of 1.2% for current smokers and 1.7% for former smokers to be an appropriate cut-off.19 Progression in emphysema and decrease in FEV1 was defined using the 95th percentile of change in nonsmokers and thresholds that define progression resulted to be an increase of 0.25% emphysema per year and a decline of 89 mL FEV1 per year. Sensitivity analyses using the 90th and 97.5th percentile showed similar patterns of statistical significance meaning the results are robust to threshold choice. An increase of 0.25% emphysema per year may be subtle to be detected by CT, but it does highlight the effect of ageing on lung density.20,21
The mean decline of 31 ml per year in FEV1 in nonsmokers in this study was comparable with other population-based studies and results are consistent with data from the Framingham Heart Study in which the upper limit of normal of lung function decline in 401 healthy adults was shown to be 101 mL per year over 4 years (83 mL per year over 7 years in 354 participants).22 Although the results of sensitivity analyses were largely robust to the choice of threshold for defining progression, using the 90th percentile of change seen in non-smokers or a 10% decline from baseline to 5-year follow-up to define FEV1 progression (and, therefore, a slightly lower threshold) might be more appropriate as this resulted in more clinically significant associations especially in GOLD 0 and GOLD 2.
In studying participants at risk of progression, we provided information on the linear relationship between clinical variables, the percentage emphysema at baseline, and changes in emphysema over time. We confirmed that a higher age, male gender, non-Hispanic white race, and current smoking were associated with higher emphysema in almost all GOLD stages.23-26 We found that in participants without COPD, males have higher emphysema at baseline and they progress faster with 0.3% more per year as compared to females. Also, in participants with GOLD 2 to 4, lower BMI played a role in emphysema progression, which is in line with results from Bhavani et al.27 In addition, more pack years were associated with higher baseline emphysema in GOLD 1 and GOLD 2, but not in the other GOLD stages. In participants without spirometric COPD and in participants with severe COPD (GOLD 3 and GOLD 4), smoking status seemed to predominate.
A few limitations need to be addressed. First, the multi-center design of the study introduced possible sources of variability, including differences in CT scanner make and model. This was addressed by including study center in the analysis, however, the measurements could still be vulnerable to other sources of noise.28,29 Although FRC/TLC was included in the linear multivariate analyses to correct for changes in inspiration and expiration level, it could be more desirable to have spirometric control or to include plethysmographic data. Second, expiratory CT measurements would ideally be measured at residual volume which is more traditionally used to assess small airway disease. However, expiration to functional residual capacity is thought to be more reproducible, and previous work has shown that parametric response-mapping metrics obtained using this technique correlate well with other functional measurements and predict decline in FEV1.6,30 Third, this analysis was limited to the first 5000 COPDGene participants who returned for a 5-year follow-up and who had acceptable CT studies. A selection bias may have been introduced due to loss of participants from illness or death. The absence of these ill or deceased participants may have reduced the signal for disease progression in this study, particularly in those with more advanced disease.
It has been shown that in current and former smokers who do not meet a spirometric definition of COPD, lung abnormalities as well as clinical impairments are common,2 but data was lacking on how to identify those who are at risk for disease progression. As emphysema is associated with increased mortality, it is important to identify those individuals who are vulnerable to cigarette smoke.31 We concluded that CT-derived emphysema and fSAD are both important in identifying individuals prone to develop progressive emphysema, which supports the potential role of quantitative CT in heavy smokers.
Acknowledgements
The project described was supported by grants R01HL089897 and R01HL089856 from the National Heart, Lung, and Blood Institute. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board representing AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline. In addition, this project has been financially supported by the Foundation “De Drie Lichten” in the Netherlands, the Lung Foundation Netherlands (grant 9.1.15.067FE), and the Foundation “Stichting Astma Bestrijding”.
Author Contributions: Study conception and design was provided by EP, CMM, JDC, EAR, and DAL. Data analysis and interpretation was provided by EP, CMM, JDC, EAR, MJS. DAL EP, CMM, FAAMH. PAdJ, J-PC, MKH, SMH, CRH, CJG, EKS, JDC, GRW, EAR, BM, MJS, J-WJL, EMvR, and DA drafted the manuscript for important intellectual content.
Declaration of Interest
E Pompe reports personal fees from Thirona BV outside the submitted work. JP Charbonnier reports personal fees from Thirona BV, during the conduct of the study. MK Han reports personal fees from GSK, Boehringer Ingelheim, AstraZeneca, and Novartis, outside the submitted work. SM Humphries reports grants from the National Heart, Lung, and Blood Institute, during the conduct of the study. CR Hatt is a salaried employee of Imbio, LLC. CJ Galbán reports grants from the National Institutes of Health, during the conduct of the study. He has a patent for the Voxel-based analysis of registered medical images acquired from multiple phases with royalties paid to Imbio. EK Silverman reports grants from the National Institutes of Health and grants and other expense payments from the COPD Foundation, during the conduct of the study. In addition, he reports personal fees from GSK, Merck, and other expense payments from Novartis, outside the submitted work. GR Washko reports support from GSK, PulmonX, and Boehringer Ingelheim, outside the submitted work. B Make reports support from Novartis for research for this study, medical advisory boards for Forest, Spiration, AstraZeneca, Boehringer-Ingelheim, CSL Bering, GSK, Novartis, Sunovian, Theravance, and Verona. In addition, he received research grants, controlled by National Jewish Health, from AstraZeneca, Boehringer-Ingelheim, Forest, GSK, Pearl, Sunovian, and the National Heart, Lung, and Blood Institute and he provided continuing medical education for the American College of Chest Physicians, the Cleveland Clinic, Consensus Medical Education, Hybrid Communications, Integrity Medical Education, Mt. Sinai Medical Center, WebMD, the Foundation for Improving Patient Outcome, Medscape, National Jewish Health, the Peer Review Institute, and SPIRE Learning. He also receives royalties from Up-To-Date, the Data Safety Monitoring Board for Spiration, the National Heart, Lung, and Blood Institute, and Baxalta. MJ Strand reports grants from the National Heart, Lung and Blood Institute, during the conduct of the study. J-WJ Lammers reports grants from TiPharma and EU, during the conduct of the study. EM van Rikxoort is co-founder and a shareholder of Thirona BV. DA Lynch reports grants from the National Heart, Lung, and Blood Institute, during the conduct of the study, personal fees from Parexel, support from Veracyte, and personal fees from Boehringer Ingelheim and Genentech/Roche, outside the submitted work. CM Moore, FAA Mohamed Hoesein, PA de Jong, JD Crapo, and EA Regan, have nothing to disclose.