Running Head: ELLIPTA EMM and App Engagement within COPD PPRN
Funding support:This study was funded by GlaxoSmithKline plc., (HO-18-19715/210024); the study used electronic medication monitors/app provided by Propeller Health.
Date of Acceptance: August 31, 2021│ Published Online: September 10, 2021
Abbreviations: electronic medication monitor, EMM; chronic obstructive pulmonary disease, COPD; standard deviation, SD; application, app; ELLIPTA EMM Experience in COPD, ESSENCE; Propeller Health, PH; Patient-Powered Research Network, PPRN; metered-dose inhaler, MDI; COPD Assessment Test, CAT; Global initiative for chronic Obstructive Lung Disease, GOLD; modified COPD treatment ratio, mCTR; body mass index, BMI
Citation: Yawn BP, McCreary GM, Linnell JA, et al. Pilot study of a patient experience with an ELLIPTA inhaler electronic medication monitor and associated integrated system: a prospective observational study using the COPD Patient-Powered Research Network. Chronic Obstr Pulm Dis. 2021; 8(4): 488-501. doi: http://doi.org/10.15326/jcopdf.2021.0218
Online Supplemental Material: Read Online Supplemental Material (363KB)
Introduction
Digital self-monitoring platforms with electronic medication monitors (EMMs) and linked smartphone applications (apps) for inhaled medications can remotely monitor and report patient inhaler use.1 EMMs attached to the inhaler automatically collect data via Bluetooth when the inhaler is actuated, tracking the timing and quantity of medication actuations.1-3 This passive data collection requires no action on behalf of the patient to track data; active data collection would require active participation from the patient to collect data. Smartphone apps linked to the EMM allow patients to review inhaler use and set up reminders for inhaler use, including for missed doses, based on their self-recorded medication schedule.1-3 Some systems also offer educational strategies to patients based on measured adherence and rescue inhaler use.1 Patients can enter their daily symptom data into the app. In some cases, the system can also provide data for environmental factors (such as air quality) that may alter symptoms.1,4,5
Adherence to inhaled therapies is important for reducing symptom burden, hospitalization, and mortality in people with chronic obstructive pulmonary disease (COPD).6 Tracking rescue inhaler use can highlight increased reliance on quick reliever therapy, suggesting the need to reassess current therapies, including adherence to maintenance medication and inhaler technique.7 Daily use of short-acting bronchodilators is not a recommended component of COPD pharmacotherapy8 and greater use of rescue inhalers is associated with increased risk of exacerbations and greater COPD burden.9,10 In people with asthma, EMM use has been associated with increased medication adherence, decreased rescue therapy use, and decreased symptom burden.2,5,11,12 People with asthma have reported EMMs as beneficial for self-management, through engagement with the associated app that allows tracking of medication adherence through inhaler use.1,11,13-15 However, few studies have included people with COPD and no studies have assessed EMMs and dry powder inhalers in people with COPD.7,16-20
The aims of this pilot study (ELLIPTA EMM Experience in COPD [ESSENCE]) were to determine the level of participant engagement with the EMM and associated app among individuals with COPD using the ELLIPTA inhaler, and to assess adherence to daily ELLIPTA inhaler-delivered maintenance medication and rescue medication use. In addition, participant experience with the EMM and app was assessed using surveys and a focus group. The study was a collaboration between the COPD Foundation, Propeller Health (PH), and GlaxoSmithKline plc.
Methods
Study Design and Setting
This was an open-label, single-arm, prospective observational cohort pilot study following participants for a minimum of 12 weeks and a maximum of 24 weeks post-index date (Figure 1). Participants with COPD were recruited from the COPD Foundation's Patient-Powered Research Network (COPD PPRN), an online registry of individuals who consented to be contacted and participate in research. All participants in this study were residents of the United States with self-reported COPD and were recruited from April 15, 2019, to July 20, 2019. Data were collected up to October 20, 2019.
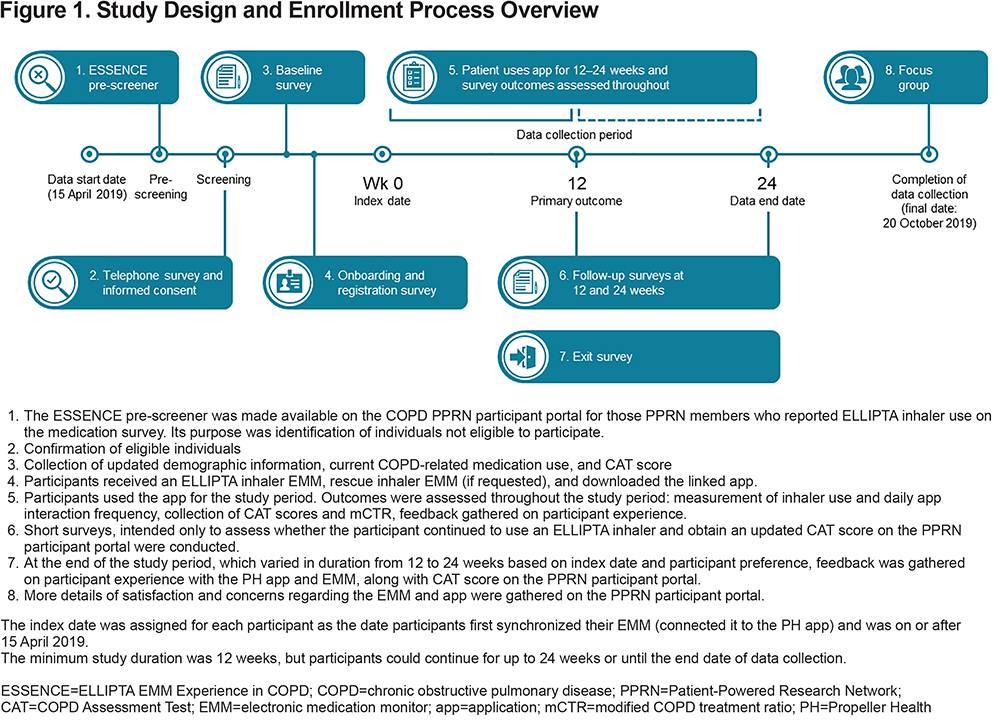
The study was approved by the Western Institutional Review Board for the COPD Foundation, and informed consent was obtained from all participants at the time of enrollment using e-consent on the COPD PPRN platform. After consenting, participants were shipped an EMM for the ELLIPTA inhaler, and could also request an EMM for their rescue metered-dose inhaler (MDI). When employing the EMM, rescue MDIs could include albuterol or a combination of ipratropium bromide and albuterol sulfate (Combivent). Once the EMM was received, participants followed the online instructions to register with PH, and then install and synchronize the EMM with the app. The index date was assigned for each participant as the date they first synchronized their EMM (i.e., connected it to the PH app). The PH platform allowed participants to observe their maintenance medication adherence and inhaler use on the app, based on the date and time of inhaler use collected by the EMM. Participants were not required to view their data but may have received a notification prompt if they had not used their maintenance inhaler in more than 4 days or if they had a significant increase in rescue inhaler use above baseline. Participants also received audio/visual maintenance medication reminders according to their self-recorded medication schedule. Participant reminders to take daily maintenance medication did not require use of the app. Participants could also receive feedback on daily and as-needed inhaler medication use and had access to evidence-based COPD-related information on triggers and symptoms on the app. A more detailed description of the PH EMM can be found in the online data supplement.
Data Source and Variables
Self-reported survey data, including COPD Assessment Test (CAT) score and information regarding continued use of their ELLIPTA inhaler, were collected from participants at baseline, 12 weeks, and up to 24 weeks or at study exit, whichever occurred earlier. The EMM was linked via Bluetooth to the PH app and collected daily inhaler usage data on inhaler actuations from the EMM connected to participants’ ELLIPTA inhaler(s) and, if applicable, from the rescue MDI EMM. Data related to participants’ daily interaction with the app were also recorded, e.g., was the app opened and if so, for how long was the app open to enter symptom data, and/or view educational information on COPD. Data were collected until the participant exited the study due to ending use of their ELLIPTA inhaler, study withdrawal, study completion at 24 weeks, or until October 21, 2019 (defined as the last date of data collection).
Participants
Participants were identified from a previously administered online medication survey that asked COPD PPRN members to report their current COPD medication use. Participants were invited to participate in a pre-study survey to determine eligibility if they reported use of one of the following ELLIPTA inhaler delivered medication: BREO, ANORO, INCRUSE or TRELEGY. Therefore, every mention of an ELLIPTA inhaler in this paper refers to one of these licensed products. Inclusion criteria were: ≥40 years of age, self-reported diagnosis of COPD, self-reported treatment with a medication delivered by the ELLIPTA inhaler, use of a smartphone (required for EMM and app connectivity), and willingness and availability to participate for ≥12 weeks. All participants provided informed consent and agreed to the PH terms of use. Participants received monetary compensation for study participation. Individuals reporting active participation in any other COPD research study or with self-reported off-label use of any ELLIPTA inhaler were excluded. The target recruitment sample was 100 participants, with 122 participants enrolled.
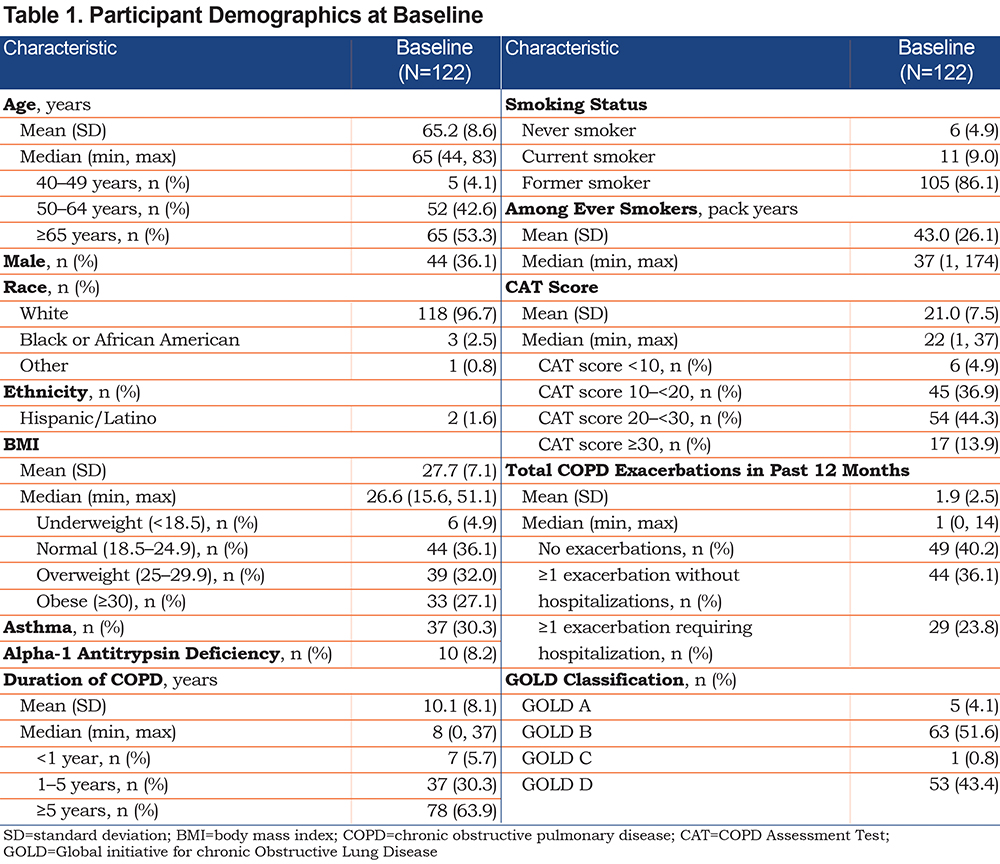
Baseline variables collected at the time of study entry included age, sex, race/ethnicity, duration of COPD, COPD pharmacotherapy at time of enrollment, and COPD clinical characteristics, including CAT score and participant-reported COPD exacerbation history in the prior 12 months (Supplementary Table 1 in the online supplement). The CAT and exacerbation history were used to classify participants into Global initiative for chronic Obstructive Lung Disease (GOLD) groups A through D.
Outcomes
The primary outcome was participant engagement with the PH app, as measured by daily active use (number of days the app was opened/days enrolled in study), during the 12 weeks post-index. Additional outcomes were measured beyond 12 weeks and up to 24 weeks post-index:
- daily active use,
- adherence rate to “as prescribed” ELLIPTA inhaler delivered maintenance medications,
- rescue inhaler use (proportion of days of use, daily frequency, and calculated rescue-free days measured among those requesting an EMM for rescue inhalers),
- CAT scores (at baseline and at 12 weeks and up to 24 weeks post-index),
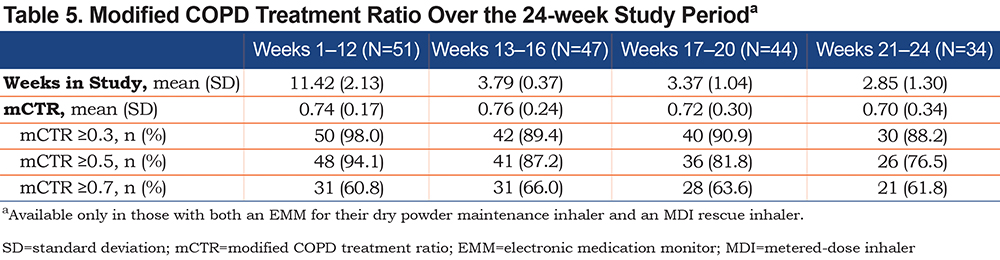
- (for the subgroup with a rescue inhaler EMM) the modified COPD treatment ratio (mCTR) (defined as the ratio of the number of actuations of the ELLIPTA inhaler to the number of actuations of the rescue inhaler plus the number of actuations of the ELLIPTA inhaler), based on EMM data in the subgroup with both ELLIPTA and rescue inhaler EMMs (e.g., 51/122 [42%]), to assess maintenance medication use in the context of rescue therapy.
Quantitative and qualitative information on participants’ insights and feedback on their experience with the ELLIPTA EMM and PH app (including acceptability, ease-of-use and satisfaction) was collected at study exit and from a focus group at close of study.
Data Analysis
Daily active app use was calculated using the number of days in which participants opened the PH app and interacted with 1 or more available sections of the app. When the app was not opened or the app was opened but no sections of the app were accessed, daily active use was recorded as 0 seconds. The EMM collected daily actuations from the ELLIPTA inhaler to determine adherence, captured as “as prescribed (days with exactly 1 actuation)” or “any other use (days with 0 or >1 actuation).” Daily active use and adherence data were also stratified by participants’ baseline CAT score,21 grouped as CAT scores of 0–9, 10–19, 20–29 and ≥30. In addition, EMMs on rescue medications captured daily utilization as a surrogate for symptoms.
Participants completed an exit survey when leaving the study (between 12 and 24 weeks) to collect feedback on their experience with the PH app and EMM, and to complete an additional CAT. Participants who continued beyond 12 weeks also completed a survey at 12 weeks. The survey questions and responses are outlined in Supplementary Table 2 in the online supplement. Responses to the survey questions were graded on a scale of 1 (low) to 5 (high). After the study, interested participants (n=12) were invited to participate in a web-based, private, asynchronous focus group using the COPD Foundation’s COPD360social online community platform. These participants were selected according to low, medium, and high engagement with the app based on quartiles of daily active use for the first 12 weeks. The focus group included prompted questions regarding satisfaction and concerns with the EMMs and app in a free-response format. The exit survey and focus group covered some overlapping topics, and the focus group was used to gather more in-depth responses. All dialogue was transcribed and summarized by the COPD Foundation.
Statistical Analysis
Data were analyzed using SAS software, Version 9.4 (SAS Institute, Cary, North Carolina). Data were censored at the time when individual participants were no longer using any medication delivered via an ELLIPTA inhaler, when participants withdrew from the study, or at the pre-determined study end (between 12 weeks and up to 24 weeks post-index date). All available data were included in the descriptive analyses.
Participant characteristics were measured as mean, standard deviation (SD), median, and range for continuous variables, and frequency and proportion for categorical variables. Summary statistics were used to report participant engagement (mean, SD, median, interquartile range, frequency, and proportion), adherence to maintenance medication and use of rescue inhaler (frequency, proportion), and symptom control and mCTR (mean, SD, frequency, proportion). Daily active app use was evaluated at 3 time periods: Days 1–30, Days 31–60, and Days 61–90. CAT scores were analyzed at 3 time points: baseline, Week 12, and at study exit. mCTR values were computed and assessed at 4 time periods: Weeks 1–12, Weeks 13–16, Weeks 17–20, and Weeks 21–24.
During data analysis, we observed that the daily recorded times for the app being open exhibited a skewed bimodal distribution, with some instances of use as long as 10 to 20 hours. This prolonged interaction with the app appeared unlikely. We, therefore, conducted a post hoc analysis excluding the upper 5% of times that the app was recorded as open.
Results
Participants
There were 1374 respondents, of nearly 7600 patients registered in the COPD Foundation’s COPD PPRN, to a COPD medication use survey in 2019. Of these, 238 reported use of 1 or more ELLIPTA inhalers for maintenance therapy and were invited to participate in a survey to determine eligibility for this study. A total of 122 participants were included in the final study population. Participant demographics and characteristics at baseline of the final study population are summarized in Table 1. Overall, the population was largely female (63.9%), had a mean age of 65 years, was White (96.7%), non-Hispanic (98.4%), and classified as GOLD Stage B (51.6%) or D (43.4%). The COPD PPRN population showed a similar proportion of female patients (60.9%) and mean age (63 years), however, a smaller proportion of the PPRN population was White (86.9%). Over 95% of the study participants had smoked, with an average 43 pack-year history, and approximately 10% continued to smoke. Participants had a mean duration of COPD of 10 years and approximately one-third of participants reported concurrent asthma. Participants’ COPD pharmacotherapy at baseline is reported in Supplementary Table 3 in the online supplement
Primary Outcome
Participant engagement with the PH app decreased over the assessment period (Table 2). The mean (SD) proportion of days in which participants interacted with the app decreased from 51.9% (30.4) for Weeks 1–12 to 46.1% (35.5) for Weeks 13–24. The time spent using the app each day also decreased over the study period; for those days on which the app was open, mean use reduced from 9.0 minutes/day for Weeks 1–12, to 6.6 minutes/day for Weeks 13–24 (Table 2). The post hoc analyses removing the upper 5% of times the app was open showed the same decreasing trend in daily app usage (minutes/day) over time. When daily active use and time the app was open were stratified by baseline CAT scores, the percentage of days the app was opened varied little by CAT score, but the participants with the highest CAT scores (those with highest symptom burden) typically spent less time using the app (Supplementary Table 4 in the online supplement).

Other Outcomes
Adherence to ELLIPTA Inhaler Delivered Maintenance Therapy
Adherence to the ELLIPTA inhaler delivered maintenance medication as prescribed (mean percentage of days [SD]) was 80.8% (22.1) for Weeks 1–12 and 71.5% (28.5) for Weeks 13–24, for an average adherence over the study period of 77.4% (21.5) for Weeks 1–24 (Table 3). Adherence to maintenance therapy was lowest in the group with CAT scores >30 and highest for those with CAT scores <10 (Supplementary Table 5 in the online supplement).
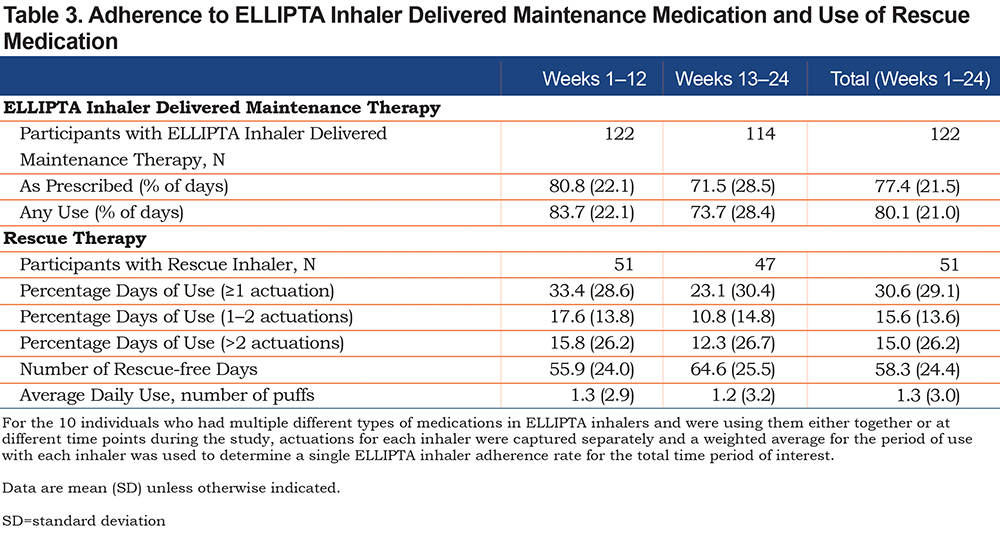
Use of Rescue Inhaler
For the subgroup with a rescue therapy EMM (51/122 [42%]), the mean percentage (SD) of days with 1 or more actuations of rescue therapy decreased from 33.4% (28.6) for Weeks 1–12 to 23.1% (30.4) during Weeks 13–24 (Table 3). This corresponded to an increase in mean (SD) rescue-free days from 55.9 (24.0 [Weeks 1–12, n=51]) to 64.6 (25.5 [Weeks 13–24, n=47]).
Symptom Control and Modified COPD Treatment Ratio
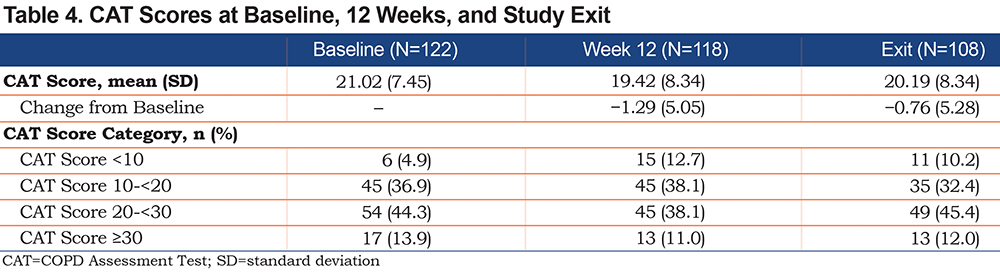
No substantial changes were seen in the mean CAT score (Table 4) over the study period. The changes from baseline in CAT score did not meet the 2-point minimal clinically important difference22 at 12 weeks (−1.29) or at 24 weeks/exit (−0.76). For the subgroup who had both a maintenance and a rescue therapy EMM (n=51), approximately two-thirds of individuals had a mCTR of ≥0.7 (representing greater use of daily maintenance therapy and lower number of days of use of rescue therapy), and this number remained stable throughout the study period (Table 5).
Exit Survey and Focus Groups
Data from the exit surveys showed that participants (N=117) were overall very satisfied with the EMM and app, with no mean score being less than 4 on the 5-point scale of satisfaction. Participants also expressed confidence in EMM use (mean 4.6 [SD 1.1]), value of the app reminders (mean 4.3 [SD 1.1]), satisfaction with the system (mean 4.1 [SD 1.1]), and ease of EMM and app use (mean 4.6 [SD 1.1] and mean 4.6 [SD 0.8], respectively) using a 5-point scale of low (1) to high (5) (data not shown).
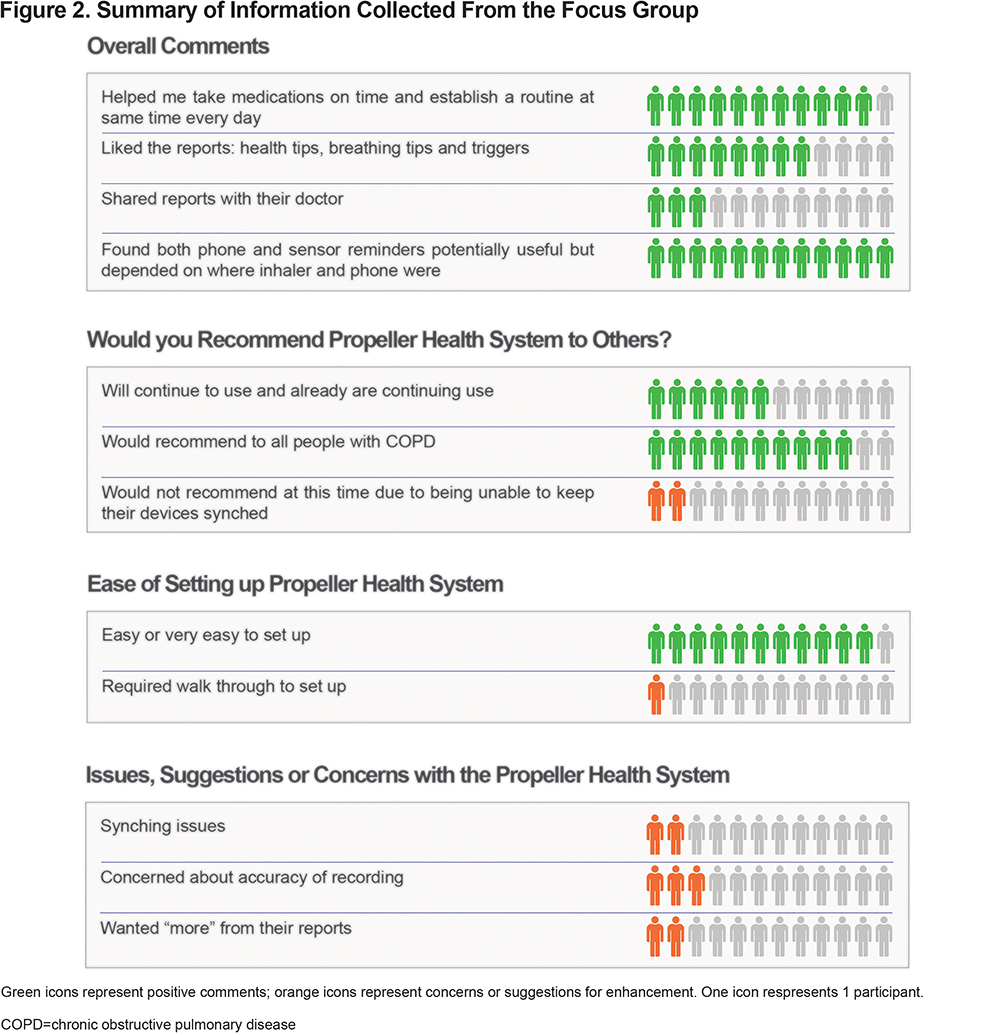
Overall, the focus group participants (N=12) were very positive about the EMM and the app (Figure 2). Most participants liked the reminders to use their medications (mean 4.3 [SD 1.1]; scale: 1=low, 5=high) and thought the reminders helped them take their medications at more consistent times during the day (mean 4.3 [SD 1.1]). The reminders seemed to be most valued by participants who lived alone. Most participants found the EMM and app easy to set up and use but 2 of the 12 participants experienced synchronization issues; they were unsure if this issue was due to their own internet or systems issues. Participants suggested more in-depth and newer COPD-related information be added to the app. Some participants (n=3) said they received reminders even after they knew they had taken their medication for the day. Therefore, other suggestions for change included a review of the design of the reminder system. Participants also suggested adding a built-in notification to remind users not to throw away the EMM when the inhaler was empty.
Discussion
Remote monitoring for chronic diseases and the availability of clinical apps for both health care professionals and patients to support patients’ care have been used increasingly in recent years for respiratory diseases such as asthma,2,11,23,24 but reported data for COPD are more limited.7,16-20 In this open-label, prospective observational pilot study of participants with self-reported COPD from the COPD PPRN registry, the EMM and associated app for monitoring the ELLIPTA inhaler delivered maintenance therapy were well received in the total population. There was frequent but decreasing app use over the study period, and high reported satisfaction and ease-of-use. Adherence to maintenance medication was high and symptom burden as assessed by the CAT was stable throughout the study. The absence of a control group made the benefit of the EMM on these parameters difficult to assess, but the constant high level of adherence noted in this study is promising.
A limited number of studies of EMM use in people with COPD, including with a dry powder inhaler, have reported satisfaction, adherence, and daily active use similar to our own findings, but none of these studies assessed the use of an EMM and associated digitized app with a dry powder inhaler, such as the ELLIPTA inhaler.25,26 High participant satisfaction and ease-of-use with an EMM device used with maintenance and/or rescue medication was reported in 2 small COPD cohorts.17,20 Adherence to maintenance therapy has been shown to increase significantly with use of an EMM and electronic reminders.18 One small study, however, reported a decline in adherence to controller inhalers over 1 year, but adherence in this study was higher than the adherence rates for people with COPD reported in the literature.20 In addition, Chen et al (2019), who assessed rescue medication use with an EMM in 190 participants with COPD, reported a decrease in rescue medication use and an increase in rescue-free days.19
The initial rates of daily app use and time spent on the app decreased over the 12-week assessment period. One possible contributor to this may have been that the app did not sustain prolonged and repeated engagement over time. This is a common occurrence with many apps and the session persistence of medical apps with intended end-users has been predicted to decrease.27,28 We hypothesize that the observed decrease in app use might also have been due to greater familiarity with the app and its available resources after the initial weeks, perhaps with a concurrent improvement in focused app use and navigation. Alternatively, decreased app use may have been associated with a change in behavioral factors, culminating in reduced app engagement. The app also includes evidence-based educational materials and the ability to review medication use patterns and record triggers and symptoms. Participants may have felt that they did not need to review these educational materials repeatedly or view patterns daily, especially if symptoms were stable, explaining the noted decreased interaction with the app. This suggestion is supported by findings from the focus group; while most found the educational materials helpful, a few (2/12) focus group members felt the materials should be enhanced since it was “information we already knew” and, therefore, did not require repeated viewing in the app. Patient reminders to take daily maintenance medication did not require use of the app and the decrease in app use did not correlate with the consistently high level of daily maintenance adherence. In a previous trial, use of a mobile app plus remote clinician feedback maintained high baseline levels of adherence to EMM use.29 However, clinicians were not involved in the current study and, therefore, no clinician feedback was provided.
Data from both the exit survey and the focus group interactions revealed that participants found the EMM and app easy to use and valuable. However, 2 members of the focus group did express challenges with the need to repeatedly synchronize their inhalers with the app. Repeated synchronization should not be required and the reasons for this included closing the app, storage of the EMM outside of Bluetooth range, and EMM battery failure. Focus group members also reported that it was ideal to have the smartphone (running the app) and inhaler together in the same location so that a reminder could be followed by immediate inhalation rather than requiring the user to go “up or down stairs” to take the inhaler after being reminded. Similarly, focus group members cited the need to ensure the smartphone battery was charged.
Many remote monitors designed for use in COPD or other chronic diseases address common problems, such as the low rate of adherence to prescribed medications, which may impact outcomes.6 Forgetting to take a medication is a common reason for poor adherence and is addressed by apps with reminder systems.30 In the PH platform there are 2 reminder systems: 1 audio reminder through the EMM and 1 visual reminder sent through the app to the smartphone. Adherence to inhaled maintenance therapy when using an EMM with electronic reminders was shown to increase in one 6-month study of patients with COPD,18 but was found to gradually decline in a separate 12-month study.20 Studies in patients with asthma have reported increases in adherence with use of EMMs.1,15,31 Observed adherence in the first 7 days of our pilot study was notably higher than average COPD maintenance medication adherence rates previously reported in the United States.32 Mean adherence to daily maintenance medications was 80% during the first 30 days and this level was maintained over the 3–6 month observation period. The absence of a control arm or a baseline adherence rate in this study, combined with the high initial adherence rate, likely made it difficult to observe improvements over time in this small pilot study. However, unlike studies requiring participant self-reporting of medication use, the EMM data provide objective confirmation of actual adherence rates. Targeted selection of participants for EMM and app use, such as those with lower rates of adherence, in conjunction with addressing causes of poor adherence, might lead to better outcomes such as lower symptom burden (e.g., CAT scores) or fewer exacerbations.7 Optimal adherence requires correct inhaler technique and the availability of EMM systems that provide feedback on the frequency of inhaler use may aid some patients and help to address the prevalence of some inhaler errors observed among patients with COPD.1,33-36 EMMs and associated apps may, therefore, contribute to an individualized treatment approach in COPD by addressing specific treatable traits such as poor adherence and incorrect frequency of inhaler use. However, cost-effectiveness studies for these devices are still required.36
Strengths and Limitations
The population for this study was a unique population, limiting the generalizability of these results to the general U.S. COPD population37; the population was largely female, showed higher smoking rates than the general COPD population, and included very few non-White participants. The cohort comprised individuals with self-reported COPD who were using an ELLIPTA inhaler, representing a potential for self-reporting bias. Selection bias may have been introduced by only including participants recruited through the COPD PPRN; this required prior online registration, favoring more technologically inclined individuals. Participants could potentially have been more motivated and involved in their care compared to the general population of people with COPD, representing self-selection bias. Participants in this study provided self-reported data, which could have been influenced by self-reporting bias, arising from recall bias.38 However, the self-reported data provided a wide range of responses, for example, on participants’ perspectives and opinions of the EMM and app, which may not have been possible with other data collection instruments. Normally, health care professional dashboards are also employed to monitor patients’ inhaler use; the lack of such a dashboard within this study may have affected outcomes since only patient self-monitoring and self-reporting were available without support of clinician interpretation or feedback. COPD PPRN participants were compensated for study participation (per the protocol approved by the Western Institutional Review Board for the COPD Foundation), but their initial involvement in the COPD PPRN was voluntary. Although this may be a potential limitation, access to the PH EMM and app may benefit many participants at no cost to them. While the 24-week study period (12 weeks for the primary outcome) might be considered relatively short, and, thus, may not be representative of longer-term EMM and app use, this was a pilot study conducted in a real-world setting following a well-characterized COPD population.
Conclusion
The EMM plus app were associated with high participant satisfaction and frequent but decreasing use of the app over time. Participants had high and stable levels of EMM-reported adherence to ELLIPTA inhaler delivered maintenance medication use. For people with COPD with a rescue EMM, the number of rescue-free days increased. Use of the PH platform may be an opportunity for greater self-management of COPD. Further work in a larger population that may have less access to and experience with online technology, greater racial and ethnic diversity, and a wider range of symptom burden is necessary to enhance generalizability of the results.
Acknowledgments
The authors thank the study participants and study team for their contributions to the study. The authors are also grateful to Ruth Tal-Singer, PhD (GlaxoSmithKline plc., at the time of the study, currently COPD Foundation), who was instrumental in the collaboration with the COPD Foundation and supported the development of the study concept.
Trademarks are owned by or licensed to their respective owners (the GlaxoSmithKline group of companies [CAT, ELLIPTA] and the Bluetooth Special Interest Group [Bluetooth]).
Author contributions: Barbara P. Yawn, John A. Linnell, Gretchen M. McCreary, Cara B. Pasquale, Elisha Malanga, David A. Stempel, Rahul Gondalia, Leanne Kaye, Ryan Tomlinson, Kathryn A. Collison, Benjamin S. Wu, Richard H. Stanford, and Daniel Gratie were involved in study conception/design; Barbara P. Yawn, Gretchen M. McCreary, Cara B. Pasquale, and Elisha Malanga were involved in data acquisition; Barbara P. Yawn, John A. Linnell, Gretchen M. McCreary, Cara B. Pasquale, Elisha Malanga, Radmila Choate, David A. Stempel, Rahul Gondalia, Leanne Kaye, Ryan Tomlinson, and Benjamin S. Wu were involved in data analysis and/or interpretation. All authors were involved in writing/critical review of draft versions of this manuscript, and all approved the final version to be submitted for publication.
Data sharing statement: The anonymized aggregate data and study protocol documents from this study are available upon request from the authors pending approval of the COPD Foundation and GlaxoSmithKline plc.
Declaration of Interest Statement
Gretchen M. McCreary, Cara B. Pasquale, Elisha Malanga, and Radmila Choate have no conflicts to report. Barbara P. Yawn has attended COPD advisory boards from GlaxoSmithKline, Boehringer Ingelheim, Sunovion, and AstraZeneca. John A. Linnell is a GlaxoSmithKline Patient Thought Leader Panel Member and a COPD Foundation Wisconsin State Advocacy Captain. David A. Stempel is an employee of Propeller Health, an affiliate of ResMed, which has received funding from GlaxoSmithKline plc., and was an employee of GlaxoSmithKline plc. at the time of the study and holds shares in GlaxoSmithKline plc. Rahul Gondalia and Leanne Kaye are employees of ResMed and were employees of Propeller Health at the time of the study. Kathryn A. Collison is currently an employee of AstraZeneca and was an employee of GlaxoSmithKline plc., at the time of the study and holds shares in GlaxoSmithKline plc. Ryan Tomlinson is an employee of GlaxoSmithKline plc., and holds shares in GlaxoSmithKline plc. Benjamin S. Wu is currently employed by United Therapeutics Corporation and Daniel Gratie is currently employed by AESARA. Benjamin S. Wu and Daniel Gratie were employed by GlaxoSmithKline plc., at the time of study. Richard H. Stanford is currently employed by AESARA and was an employee of GlaxoSmithKline plc., at the time of study, and holds shares in GlaxoSmithKline plc.
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Kirsty Millar, MSc, and Andrew Briggs, BA, of Ashfield MedComms (Macclesfield, United Kingdome), an Ashfield Health company, and was funded by GlaxoSmithKline plc.