Running Head: Statin Treatment Disparities in a COPD Cohort
Funding Support: This research project is supported by cooperative agreement U01 NS041588, co-funded by the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Aging (NIA), and the National Institutes of Health (NIH), in the Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA.Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data. Additional funding was provided by the National Heart, Lung, and Blood Institute (NHLBI), grant R01HL080477 (Safford). This work is supported by the National Institutes of Health T32-HL134629, the Weill Cornell Dean’s Diversity and Healthcare Disparities Research Award, and the Donna Redel Research Fund.
Date of Acceptance: June 29, 2023 | Published Online Date: July 5, 2023
Abbreviations: ATP=adult treatment panel III; BMI=body mass index; CES=Center for Epidemiology Studies-Depression scale; CHD=coronary heart disease; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CVD=cardiovascular disease; FRS=Framingham Risk Score; HDL-C=high density lipoprotein cholesterol; LDL-C=low-density lipoprotein cholesterol; MI=myocardial infarction; PR=prevalence ratio; REGARDS=REasons for Geographic And Precise Differences in Stroke study; PCS=physical component score (of the 12-item Short-Form Health Survey)
Citation: Krishnan JK, Mallya SG, Nahid M, et al. Disparities in guideline-concordant statin treatment in individuals with COPD. Chronic Obstr Pulm Dis. 2023; 10(4): 369-379. doi: http://doi.org/10.15326/jcopdf.2023.0395
Introduction
Cardiovascular disease (CVD) is a leading cause of death in patients with COPD, with estimates identifying CVD as the underlying cause of death in 20%–30% of patients in varying COPD cohorts.1-3 Individuals with COPD are 2–5 times more likely to have major CVD.4-6 Individuals with COPD and comorbid CVD are at increased risk for COPD-related emergency department visits, hospitalization, and office visits compared to individuals with COPD alone.7
Black women with COPD have a 44% higher risk of CVD-related mortality compared to White women, driven by the disproportionate burden of CVD risk factors among Black women.8 Given the increased risk of CVD mortality among Black women with COPD, identifying disparities in the treatment of CVD risk factors in a COPD population could serve as a potentially modifiable target to improve mortality outcomes among Black individuals.
Statins are used for the prevention of CVD events and have been shown to reduce the risk of future major CVD events and to decrease all-cause mortality.9,10 Among persons with elevated low-density lipoprotein cholesterol (LDL-C) in the general population, Black individuals are less likely to be treated with statins than their White counterparts, even after multivariate adjustment including health insurance status and age.11 While prior data demonstrates suboptimal control of CVD risk factors in COPD,12 it is unknown whether treatment disparities seen in the general population are also present in a COPD population. Having multiple comorbidities, as is typical in patients with COPD, has been shown to lead to quality of care deficiencies.13 These deficiencies could be magnified among Black individuals living with COPD, who have known structural barriers to health care access.14-17
To quantify treatment with statins among adults with COPD, we conducted a cross-sectional analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) study cohort, which is well-suited to study health care disparities given its large size, national reach, rich baseline data, and oversampling of the Black community-dwelling population.18 Our objectives were to: (1) determine if there are differences in statin treatment between race-sex groups among those with COPD, and (2) determine if observed differences can be explained by factors influencing health services utilization. We hypothesized that White men would be the most likely to receive statin treatment compared to other race-sex groups, and this disparity would not be explained by individual factors posited to account for differences in health care utilization.19
Methods
Study Design
REGARDS is a national, prospective observational cohort study designed to examine causes of regional and racial differences in stroke mortality.18 Participants were enrolled between 2003 and 2007 using commercially available listings to recruit community-dwelling Black and White adults aged ≥ 45 living in the continental United States, with oversampling from the Black population. Baseline data were collected through computer-assisted telephone interviews assessing medical history and health status as well as in-home visits by trained health professionals. In-home visits followed standardized, quality-controlled protocols to obtain physical measurements, electrocardiograms, blood and urine specimens, and medication information via an in-home pill bottle review. All data used in our analyses were obtained from the telephone interview and in-home visits which occurred at the time of enrollment between 2003 to 2007. This study was approved by the University of Alabama at Birmingham and Weill Cornell Medical College’s institutional review boards (protocol #1603017100) and all participants provided informed consent.
Study Sample
We previously identified evidence of diagnosed COPD among REGARDS participants who were Medicare beneficiaries at the time of REGARDS enrollment.8 A diagnosis of COPD was determined using a modified previously validated algorithm for identifying COPD in administrative claims records.8,20,21 A participant was identified as having COPD if they had any inpatient hospitalization with a COPD-specific diagnosis code (International Classification of Diseases-9th Revision 491.x, 492.x, and 496) in any position or a COPD-specific diagnosis code on 2 outpatient claims from different service dates.
Among participants with diagnosed COPD, we identified those with an indication for statin for the prevention of CHD. Indication for statin treatment was defined by the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program guidelines that were current at the time of the REGARDS enrollment,22 or by already being on statin treatment, consistent with prior REGARDS studies.11,23
The ATP III criteria identify LDL-C targets based on the future risk of coronary heart disease (CHD). Individuals with a CHD risk equivalent (presence of CHD, diabetes, or prior vascular disease) are at highest risk. Prior vascular disease was detected by self-reported history of stroke, heart attack, abdominal aortic aneurysm, peripheral arterial disease, or coronary revascularization procedure or by evidence of myocardial infarction on the baseline ECG. Diabetes was present if participants were treated with diabetes medication or had a fasting glucose ≥ 126 mg/dL or non-fasting glucose of ≥ 200 mg/dL.
For those without a CHD risk equivalent, ATP III assigns points based on other risk factors which determine the 10-year Framingham Risk Score for CHD (FRS). Those with CHD risk equivalent(s) or an FRS>20% had an LDL-C goal of <100 mg/dL. The FRS 10%–20% category had an LDL-C goal of <130 mg/dL. The FRS <10% category had an LDL-C goal of <160 mg/dL, or <130 mg/dL if 2 or more of the following risk factors were present: (1) hypertension (>140/90 mmHg or on antihypertensive medications), (2) current smoking, (3) male sex, (4) age >45 years for men and >55 years for women, (5) family history of myocardial infarction at age <55 years in a first-degree male relative or <65 years in first-degree female relative, or (6) high-density lipoprotein cholesterol (HDL-C) <40 mg/dL. If a participant had an LDL-C level greater than or equal to the LDL-C goal for a particular risk category, they were determined to have an indication for statin therapy.
Primary Outcome
The primary outcome was receipt of statin therapy among those who had an indication, based on in-home pill bottle review. The in-home pill bottle review consisted of asking participants to gather all prescription and non-prescription medication taken within the last 2 weeks. These medication names were then recorded by a study staff member on a medication data collection form.
Key Explanatory Variables
The key explanatory variables were self-reported race (Black versus White) and sex (male versus female). Race and sex were analyzed together as 4 race-sex groups: Black women, Black men, White women, and White men. White men were designated the reference group since previous studies have shown that White men are more likely to be treated with statins than other race-sex groups despite similar indications.11
Covariates
Aday and Andersen proposed that predisposing, enabling, and need-related factors may influence individual health services utilization.19 Predisposing factors are characteristics of the individual or community that exist prior to the individual’s illness, such as age. Enabling factors increase the likelihood of health services usage, such as access to transportation or insurance. Perceived need factors reflect an individual’s perception that a particular health care resource is necessary. Observed need factors are those assessed by a health care professional (e.g., elevated FRS), that would lead a physician to recommend a health resource.
In the current analysis, predisposing factors included age, educational attainment (less than high school education, high school education, or above), residence in a health professional shortage area,24 residence in a state with the least public health infrastructure,25 and residence in a zip code with high poverty level (>25% of the residents living below the federal poverty line), and stroke region. Enabling factors included social isolation (reporting not having close friends or family members or not seeing them at least once a month) and a poor social network (reporting having no one to take care of them should they become ill or disabled). Perceived need factors included awareness of hyperlipidemia (reporting having been told by a doctor that they have high cholesterol or an abnormal level of fats in their blood), medication adherence (assessed by a validated 4-item scale),26 current smoking status, and physical functioning, as reflected in the 12-item Short-Form Health Survey physical component summary (PCS) score.27 Observed needs included CHD risk category, depressive symptoms as reflected by a score ≥ 4 on the 4-item Centers for Epidemiology Studies-Depression (CES-D) scale,28 HDL-C ≤ 60mg/dL, and body mass index (BMI) >30kg/m2. The presence of depressive symptoms was included as an observed need variable due to prior studies that have demonstrated an association between depression and incident cardiovascular disease.29-31
Statistical Analysis
Among individuals with diagnosed COPD and a statin indication, we described baseline characteristics among race-sex groups (Black women, Black men, White women, White men). The proportion of all individuals with a statin indication who received treatment was compared across 4 race-sex groups.
Because the primary outcome (presence of statin medication during in-home pill bottle review) was common, prevalence ratios (PR), rather than odds ratios, were estimated for statin treatment across race-sex groups using sequential Poisson regression models with robust variance.32,33 The first model included race-sex groups with White men as the reference category (Model 0). Predisposing covariates (age, educational attainment, stroke region, residence in a health professional shortage area, residence in a state with the least public health infrastructure, residence in a zip code with a high poverty level, stroke region) were added to Model 0 to create Model 1. Enabling factors (social isolation and poor social network) were added to the Model 1 covariates to create Model 2. Perceived need factors (awareness of hyperlipidemia, medication adherence, current smoking, PCS score) were added to Model 2 covariates to create Model 3. Finally, observed need factors (CHD risk category, CES-D ≥4, HDL-C ≤60mg/dL, and BMI>30) were added to Model 3 to construct Model 4. We used multiple imputation by chained equations to impute missing covariates.34 The covariates with the most missing data were the PCS score, for which 112 participants (7.8%) had missing data, and social isolation, for which 82 participants (5.7%) had missing data. The proportion of the sample with any missing information was 24%.
We also determined the proportion of individuals receiving indicated statin treatment by race and sex among the following prespecified groups: (1) participants without prior CVD history who had an indication for primary prevention to prevent the development of future CVD, (2) participants with prior CVD and who, therefore, have an indication for secondary prevention, and (3) participants age <75 years of age. We performed a subgroup analysis including only participants aged <75 years because clinical trials historically have included fewer people over 75 years of age, which may affect prescribing practices.35,36 Prior CVD was defined as self-reported history of myocardial infarction (MI) or evidence of MI on ECG, self-reported history of coronary artery bypass graft surgery, angioplasty, stenting, procedure to fix the arteries in the neck or the legs, stroke, or abdominal aortic aneurysm. All analyses were carried out using STATA, version 17 (StataCorp, College Station, Texas). The threshold for statistical significance was p<0.05.
Results
Participant Characteristics
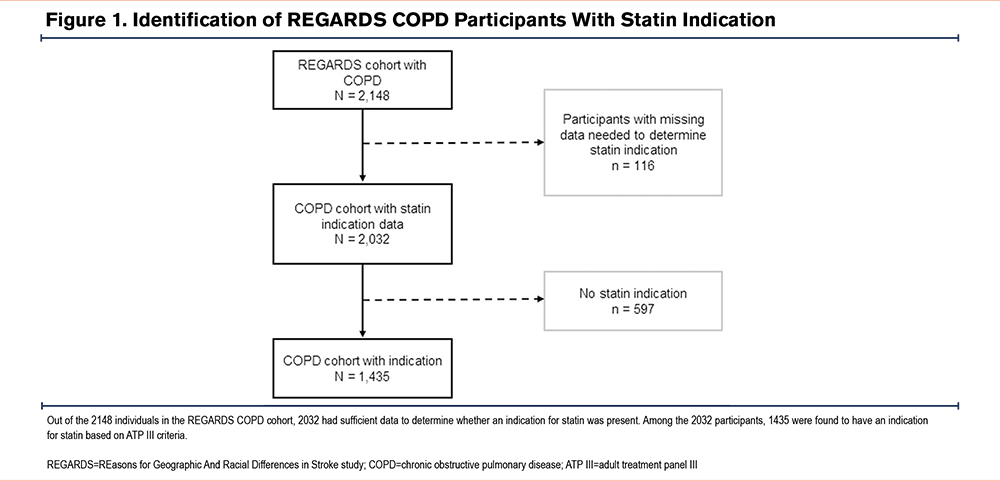
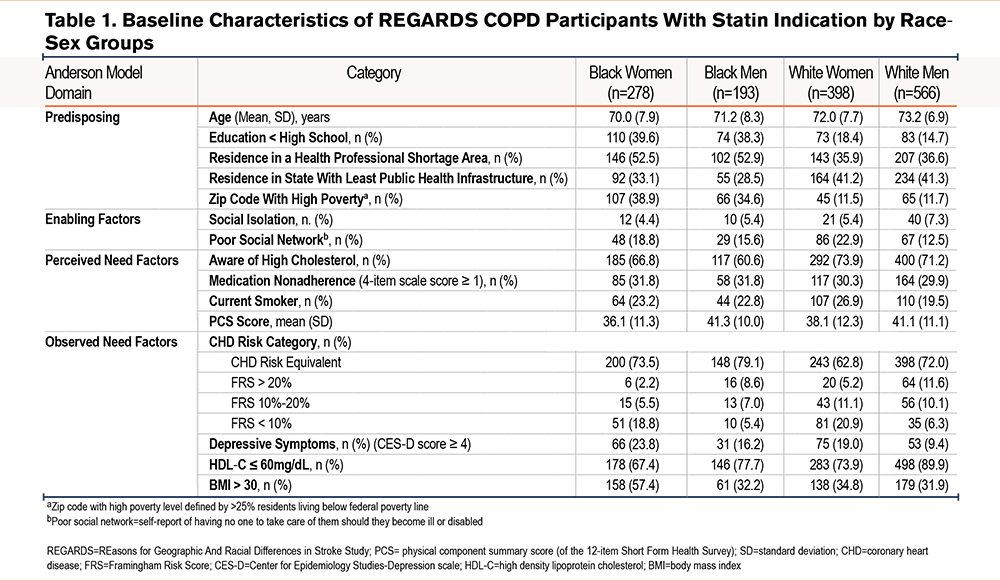
Of the 2148 REGARDS participants with a diagnosis of COPD using Medicare fee-for-service claims data at the time of enrollment in REGARDS, 116 (5.4%) participants had insufficient data to determine whether they should be on a statin. As such, these individuals were excluded. Of the remaining 2032 participants, 1435 (70.6%) had an indication for statin treatment (Figure 1). Among these 1435 participants, 19% were Black women, 14% Black men, 28% White women, and 39% White men. The characteristics of the sample by race-sex group are shown in Table 1. Black men and women were more disadvantaged when comparing several of the variables representing predisposing factors to health care utilization. When examining variables representing perceived and observed need for health services, White women comprised the highest proportion of current smokers (26.9%). Black women comprised the highest proportion of individuals with BMI>30 (57.4%) as well as depressive symptoms (23.8%). However, Black women had more favorable HDL-C levels (only 67.4% of Black women with HDL-C ≤60 compared to 89.9% of White men).
Receipt of Statins by Race and Sex
Of the 1435 REGARDS participants with diagnosed COPD and an indication for a statin, 859 participants (59.9%) were treated. Black women comprised the lowest proportion of participants receiving statin treatment (49.6%) compared to the other race sex groups (54.4% of Black men, 59.6% of White women, and 67.0% of White men) (Figure 2).
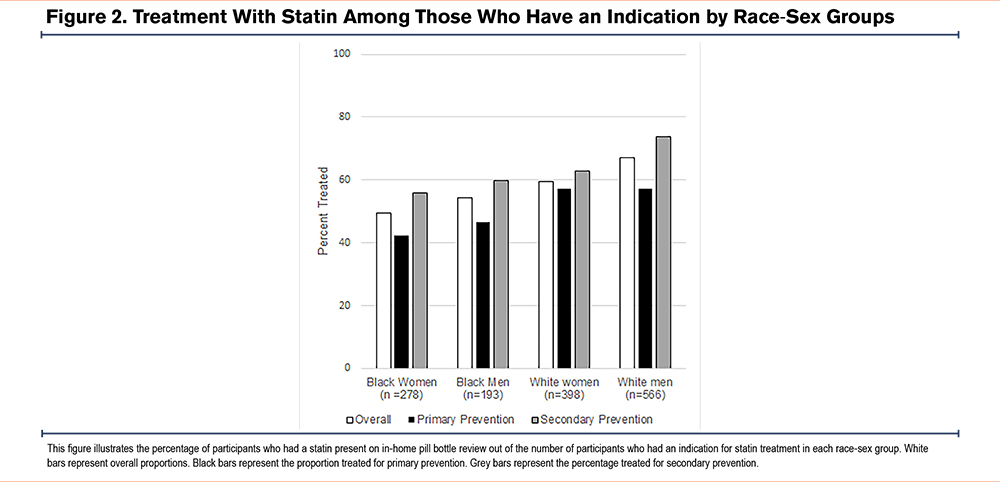
Among these 1435 participants, 593 (41.3%) had no prior history of CVD and a statin was indicated for primary prevention, and 812 participants (56.6%) had prior CVD history necessitating a statin for secondary prevention. Black women for whom a statin is indicated for primary prevention remain the least treated group. Even among the subgroup with established CVD who require statins for secondary prevention, the proportion of Black women treated was 18.1% less than that of White men (Figure 2).
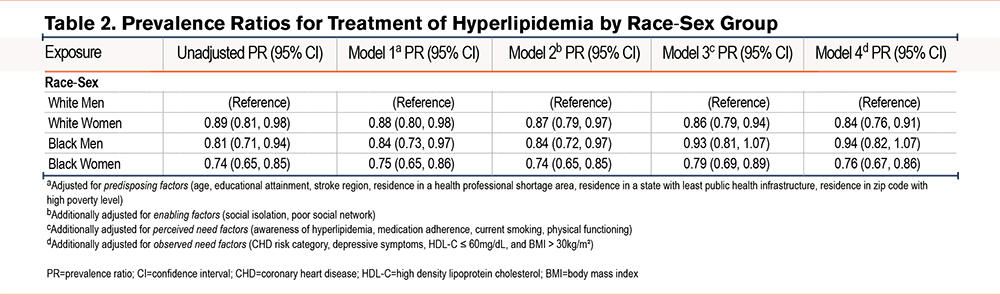
Table 2 presents the unadjusted and sequentially adjusted PRs for treatment with an indicated statin for each race-sex group compared with White men. Each race-sex group was less likely than White men to receive an indicated statin in the unadjusted model. Prevalence of treatment with statin for Black women was 26% lower than White men, (PR 0.74, [95% CI 0.65 to 0.85]) and numerically lower than other race-sex groups. After adjusting for factors influencing health care utilization based on the Andersen model, both White women and Black women had lower PRs for treatment compared to White men. In contrast, after adjusting for perceived need factors (awareness of hyperlipidemia, medication adherence, current smoking, and physical functioning), the decreased PR for statin receipt comparing Black versus White men lost statistical significance. The fully adjusted PR comparing White women versus White men was 0.84 (95% CI 0.76 to 0.91). The PR comparing Black women versus White men was 0.76 (95% CI 0.67 to 0.91).
Prespecified Subgroup Analysis
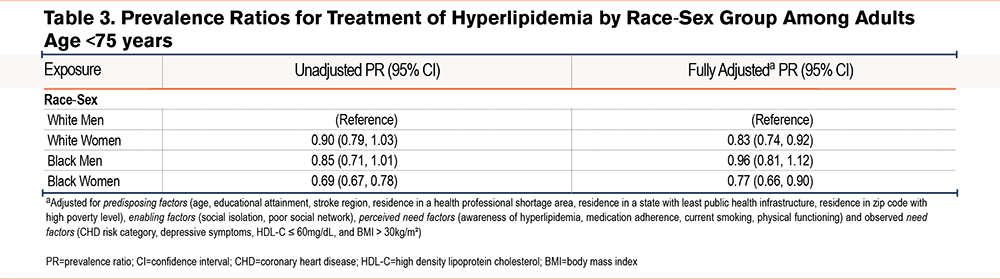
In the analysis among the subgroup that was age <75 at the time of enrollment (n=901), results were similar, with the fully adjusted model showing that White women (PR 0.83 [95% CI 0.74 to 0.92]) and Black women (PR 0.77 [95% CI 0.66 to 0.90]) were less likely to be treated with statins than White men among those who had an indication (Table 3).
Discussion
Black women with COPD are at increased risk for CVD-related mortality, but to our knowledge, no studies have assessed differences in the receipt of primary and secondary CVD prevention among persons with COPD by race and sex.8 Our results show that women, especially Black women, were less likely to receive statin treatment for both primary and secondary prevention. This difference persisted even after adjusting for predisposing, enabling, and need factors that influence health care utilization. Although there were significant differences between Black women and White men in factors that we hypothesized to influence health care utilization, such as education level and residing in a health professional shortage area, these covariates had little effect on our findings. These data suggest that changes are needed on a health system level in terms of how care is delivered to individuals with multiple chronic conditions and social disadvantages.
While the disparity in statin treatment by race is consistent with findings from the general population, the treatment gap between Black women and White men is wider in our REGARDS COPD population compared to the general REGARDS population.11,23,37 The overall difference in statin treatment between Black versus White individuals has been reported to range between 5%–15% in multiple cohorts, which is consistent with the 12% difference we identified here by race. However, when looking at differences by race and sex, an important example of intersectionality, the disparities are worse in the COPD population. An analysis of the general REGARDS population by Schroffet al identified a crude 9% difference in proportion treated between White men and Black women.23 In our COPD population, this disparity is nearly double, with a 17% difference. Despite the increased risk of CVD in the COPD population, our results suggest that disparities in therapies for CVD risk reduction in this population are even worse.4-6,38-40
The finding of worse disparities in CVD prevention within the COPD population may be due to the complexity of managing multiple comorbidities combined with barriers to accessing health care in the Black population.14-17,41,42 Our findings indicate that disparities in CVD prevention are even worse with comorbid COPD compared to diabetes, another chronic disease.43 Multiple mechanisms may influence why the disparity is worse in patients with COPD compared to the diabetes populations. Having multiple chronic conditions may decrease the likelihood of receiving guideline concordant care for each condition when they are seemingly unrelated.44 For example, patients with chronic lung disease were less likely to receive treatment for arthritis, likely due to these conditions being viewed as unrelated.13 In this case, diabetes may be more clearly perceived by clinicians to be related to CVD, as diabetes is well recognized as a major CVD risk factor.45
Another reason that comorbid conditions result in decreased guideline concordant care is that symptoms from one condition compete with another for time and attention.46 This situation is exacerbated among patients who have social barriers to care. COPD often creates symptoms of shortness of breath that may crowd out attention to preventive interventions. This study points to the need for innovative interventions to proactively optimize the management of both comorbid COPD and CVD among socially disadvantaged patient groups that are holistic and person-focused rather than disease-centric.
Our results demonstrate that Black women with COPD are at especially high risk of not receiving guideline-concordant statin treatment for CVD prevention. This is consistent with prior literature which has repeatedly demonstrated that Black women face large health inequities in multiple disease states.47 Intersectionality theory posits that Black women are particularly vulnerable as the disadvantage of having both identities is greater than the sum disadvantage of being Black and a woman. Black women experience more structural barriers to health care including racism and sexism compared to Black men or White women.48,49 Our findings highlight the need for clinicians to proactively manage comorbidities in Black women with COPD as they are particularly vulnerable to structural inequity.
The decreased likelihood of receiving a statin in Black versus White men lost statistical significance after adjusting for perceived need factors. Perceived need factors such as awareness of hyperlipidemia may play a key role in whether Black men receive statins compared to White men. Awareness of personal cardiovascular risk has been shown to influence health behaviors.49-51 Alternatively, the relatively lower number of Black men (n=193) in our cohort may have limited our statistical power or generalizability.
Study strengths include the national reach of the REGARDS cohort, oversampling of the Black population, the large number of rigorously collected covariates including in-home pill bottle review of medications, biometric data, and validated self-report variables.52 Limitations include that our COPD cohort was defined using a Medicare claims-based algorithm, a method that is susceptible to errors in omission and misclassification.20 Consistent with established approaches in CVD epidemiology, the REGARDS cohort baseline data collection focused on the detection of CHD, as defined by having an actual event such as a myocardial infarction or coronary intervention.53,54 Therefore, individuals with medically-managed coronary artery disease may not be included in the established CVD group. Additionally, the results of this study reflect relatively older data collected between 2003–2007. The cross-sectional, observational design of our study limits drawing causal inferences and capturing how management may have changed over time. Information on important factors such as mistrust of the health care system, access to high-quality care, and other forms of structural inequity, which impact health care utilization, were not available.55 While sex was a main variable for comparison, REGARDS did not collect data regarding gender identity.
In conclusion, women, especially Black women, were significantly less likely to receive indicated statin treatment compared to White men. The persistence of this disparity after controlling for individual factors influencing health care utilization suggests a need for health system-level interventions to improve receipt of statin therapy in this vulnerable population.
Acknowledgements
Author contributions: JKK, SGM, MN, ADB, MKH, KIA, PG, LCP, SB, FJM, and MMS contributed to the conception and design of the study. MMS contributed to the acquisition of data. JKK, SGM, and MN contributed to data analysis. JKK, SGM, MN, ADB, MKH, KIA, PG, LCP, SB, FJM, and MMS contributed to the interpretation of data. JKK and SGM drafted the initial manuscript. JKK, SGM, MN, ADB, MKH, KIA, PG, LCP, SB, FJM, and MMS contributed to revisions for critically important content. All the authors approved this submitted version of the manuscript.
Declaration of Interest
JKK reports support from the NIH, and the Weill Cornell Medicine Dean’s Diversity and Healthcare Disparity Research Award directly related to the submitted manuscript. She also reports financial support from the American Thoracic Society Fellowship in Health Equity, the Donna Redel Research Fund, and the Weill Cornell Research Assistance for Primary Parents Award and receipt of medication samples from Novartis, GSK, and Boehringer Ingelheim to the institution. SGM, MN, ADB, LCP, and PG report no conflicts of interest. MKH reports grants/contracts from the NIH, Sanofi, Novartis, Nuvaira, Sunovion, Gala Therapeutics, the COPD Foundation, AstraZeneca, GSK, Boehringer Ingelheim, the American Lung Association, and Biodesix. She also reports data safety monitoring board/advisory board participation for Novartis and Medtronic, leadership roles with the COPD Foundation, the American Lung Association, the American Thoracic Society, and the GOLD Scientific Committee; stocks in Meissa Vaccines and Altesa Biopharma; royalties/licenses from UpToDate, Norton Publishing, and Penguin Random House; consulting fees from AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, United Therapeutics, Regeneron, and Altesa BioPharma; honoraria from Cipla, Chiesi, AstraZeneca, Boehringer Ingelheim, GSK, Medscape, Integrity, NACE; and personal fees from Medscape and Integrity. FJM reports grants/contracts from AstraZeneca, Chiesi, GSK, Sanofi/Regeneron; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GSK, Novartis, Polarean, Pulmonx, Sanofi/Regeneron, Sunovion, Teva, Theravance/Viatris, UpToDate; honoraria from AstraZeneca and GSK; and data safety monitoring board/advisory board participation in MedTronic and GSK. KIA reports funding from the NHLBI, the Scleroderma Foundation, the American Lung Association, and the Stony-Wold Herbert Fund; and leadership roles as a member of the Pulmonary Fibrosis Foundation Scientific Review Committee and PCORI merit reviewer. SB reports a position on the data safety monitoring board for the Optimizing a Closed-Loop Digital Meditation Intervention for Remediating Cognitive Decline and Reducing Stress in Older Adults study. MMS reports support for the present manuscript with NHLBI grant R01HL80477.