Running Head: Vaccination in Alpha-1 Antitrypsin Deficiency
Funding Support: OJE received support from the Elaine Galwey Memorial Research Bursary. CHG receives support from the National Institutes of Health (P30 DK089507, UL1TR000423).
Date of Acceptance: August 31, 2023 | Published Online Date: September 6, 2023
Abbreviations: AAT=alpha-1 antitrypsin; AATD=alpha-1 antitrypsin deficiency; AEs=adverse events; AESI=adverse events special interest; CI=confidence interval; COPD=chronic obstructive pulmonary disease; DLCO=diffusing capacity for carbo monoxide; ER=endoplasmic reticulum; FDA=U.S. Food and Drug Administration; FEV1=forced expiratory volume in 1 second; IL=interleukin; IV=intravenous; NF-κB =nuclear factor kappa-light chain enhancer of activated B cells; OR=odds ratio; VITT=vaccine-induced immune thrombocytopenia
Citation: McElvaney OJ, Cleary B, Fraughen DD, et al. Safety and reactogenicity of COVID-19 vaccination in severe alpha-1 antitrypsin deficiency. Chronic Obstr Pulm Dis. 2024; 11(1): 3-12. doi: http://doi.org/10.15326/jcopdf.2023.0432
Online Supplemental Material: Read Online Supplemental Material (411KB)
Introduction
Alpha-1 antitrypsin deficiency (AATD) is an autosomal codominant disorder resulting from mutations in the pleomorphic SERPINA1 gene1,2 on the long arm of chromosome 14. The most common pathogenic allele is Pi*Z, which causes misfolding of the alpha-1 antitrypsin (AAT) glycoprotein, with subsequent polymerization and accumulation in the endoplasmic reticulum (ER).3,4 As a result, persons who are homozygous for Pi*Z display a severe functional AAT deficiency with circulating AAT levels substantially below the putative protective threshold of 11µM.5 Affected individuals exhibit dysregulated inflammatory responses and a predilection for autoimmunity,6-11 and are at significantly increased risk of developing early-onset emphysema, particularly if they smoke.12-14 They are also considered to be priority candidates for vaccination against respiratory viruses such as influenza and SARS-CoV-2, the pathogen causing severe COVID-19.
In addition to its role as the archetypal serine protease inhibitor,15-17AAT is a potent anti-inflammatory and immunomodulatory molecule,6,7,9,10,18,19 and a key component of the acute phase response.20 In critically unwell patients with the normal Pi*MM genotype, delayed or insufficient AAT production relative to acute systemic cytokinemia is associated with poor outcomes,21 and treatment with exogenous AAT reduces the circulating inflammatory burden.22 Paradoxically, in patients with Pi*ZZ AATD, rapid activation of AAT production may serve to amplify inflammation, rather than dampen it, by inducing an ER stress response as the cell struggles to clear increasing amounts of misfolded protein.23-26 The subsequent activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) drives production27 of the master proinflammatory cytokine interleukin (IL)-6. This concept applies not only to pathogenic stimuli, but also to physiological stresses such as the immune response to vaccines.
Despite the potential for exaggerated inflammatory responses following vaccination – and attendant increases in adverse events (AEs) such as fever, pain, and arthralgia – AATD-specific vaccine studies are rare.28-31 Specifically, no studies exist regarding the safety, tolerability, and reactogenicity of widely used vaccines in Pi*ZZ individuals.
ChAdOx1 nCoV-19 (AstraZeneca), a replication-deficient chimpanzee adenoviral vector containing the sequence for the SARS-CoV-2 structural surface glycoprotein antigen, was one of the first 6 vaccines to be authorized for emergency use in humans. Although early clinical studies described a favorable safety profile, ChAdOx1 nCoV-19 has subsequently been associated with rare but potentially life-threatening side effects, such as vaccine-induced immune thrombotic thrombocytopenia (VITT). In contrast, BNT162b2 (“Comirnaty,” BioNTech/Pfizer) is an mRNA-based vaccine against SARS-CoV-2 that has been administered both as an initial 2-shot vaccine series and as a booster inoculation. More recently, a bivalent version containing 2 mRNA components of SARS-CoV-2 (one from the original virus strain and the other a shared component of the BA.4 and BA.5 lineages of the SARS-CoV-2 omicron variant) has been used as an updated booster.
In this study, we prospectively assessed the incidence of vaccine-associated AEs in Pi*ZZ AATD patients undergoing the initial ChAdOx1 nCoV-19 series compared to Pi*MM individuals with COPD and non-lung disease controls receiving the same vaccine on the same day. We subsequently evaluated the safety and tolerability of sequential booster vaccination with monovalent and bivalent BNT162b2, and coadministration of BNT162b2 with the annual quadrivalent influenza vaccine, in the Pi*ZZ cohort.
Methodology
Ethical approval for the study was granted by the Beaumont Hospital Ethics Committee. Patients with Pi*ZZ AATD (n=179) and Pi*MM COPD (n=175) were consecutively identified from the Irish Alpha-1 Patient Registry, the Alpha-1 National Targeted Detection Programme, the Irish national AATD clinic at Beaumont Hospital, and the Beaumont Hospital respiratory outpatient department and offered vaccination with ChAdOx1 nCoV-19. This recruitment drive followed the inclusion of patients with COPD and AATD as priority candidates for vaccination against SARS-CoV-2 by the Irish Department of Health. Patients who accepted the offer of vaccination and provided informed consent (Pi*ZZ AATD: n=170; Pi*MM COPD: n=150) were included in the study analysis and matched with non-lung disease controls (n=140) attending for vaccination in the same week. Pi*MM and Pi*ZZ status was confirmed by immunofixation of serum glycoforms via isoelectric focusing gel electrophoresis, as previously described.32 The percentage of the predicted value for forced expiratory volume in 1 second (FEV1 % pred) was calculated using the Global Lung Function Initiative normative equations reflective of multi-ethnic reference values.33
Monitoring and Recording of Adverse Events
Each patient was monitored for vaccine-associated AEs with virtual consultations at 2-day intervals over the 8 days that followed their first dose. Consultations were conducted in a protocolized manner to minimize inter-investigator variability. AEs were classified as local or systemic, with the latter further subcategorized into common or less common. AEs of special interest (AESI) were identified in accordance with the World Health Organization definition and also recorded. AE severity was graded using toxicity grading scales approved by the U.S. Food and Drug Administration (FDA).34 The relatedness of AEs to vaccination was adjudicated by 2 separate physicians 24 hours apart. To investigate the association between prevaccination safety concerns and AE reporting, and to mitigate their potentially confounding effect, participants were also surveyed regarding their level of worry and expectation of vaccine-associated AEs at enrollment.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation unless otherwise stated. Categorical variables are summarized as counts and percentages. No imputation was made for missing data. Factors hypothesized to be associated with an increased risk of AEs were evaluated by multivariate logistic regression. Odds ratios, relative risks, and differences between groups were analyzed using Stata and graphed using GraphPad Prism 8.0 software for Windows. A value of p <0.05 was considered statistically significant.
Results
Characteristics of the Cohort at Study Entry
Baseline clinical and demographic characteristics of the Pi*ZZ cohort at study entry are available in Table 1. Pi*ZZ patients had a mean FEV1 % pred of 61.7 ± 31.6 and diffusing capacity for carbon monoxide (DLCO) of 57.4% ± 25.3%; 39 (23%) had known hepatic involvement on focused liver ultrasound. Six Pi*ZZ patients (4%) were active smokers, 99 (58%) were ex-smokers and 4 (2%) were active vapers. By comparison, the mean FEV1 %pred in the Pi*MM COPD group was 57.5 ± 22.6, with 69 active smokers (46%) and 14 vapers (9%)(Table S1 in the online supplement). Non-lung disease controls had a similar prevalence of smoking and vaping to Pi*ZZ participants (7% and 4% respectively). Although Pi*ZZ participants and non-lung disease controls were comparable in age, all Pi*MM COPD participants were over the age of 50 (Table S2 in the online supplement). The 3 study groups were comparable for sex and other known comorbidities (Tables S2, S3 in the online supplement). Fifteen AATD patients were receiving weekly intravenous (IV) augmentation therapy at a dose of 60 mg/kg. Prevaccine safety concerns were reported by over one-third of participants who accepted the offer of vaccination, with approximately 6% of the total cohort stating that, despite deciding to attend, they were “very worried” vaccination with ChAdOx1 nCoV-19 would result in severe side effects (Figure S1 in the online supplement).
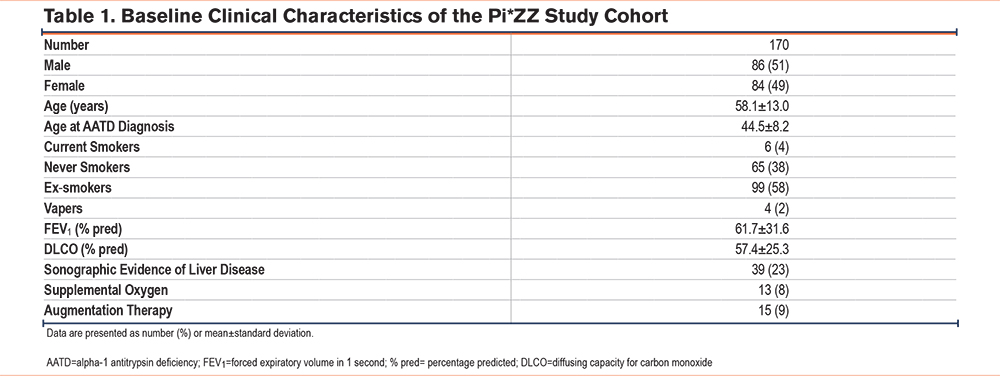
Vaccine-Associated Events Following Administration of ChAdOx1 nCoV-19
AEs were common in Pi*ZZ participants, with 138 (81%) experiencing at least one local AE and 103 (61%) reporting at least one systemic AE after their first ChAdOx1 nCoV-19 dose (Table 2). However, the proportions of Pi*ZZ participants recording AEs were not significantly different from those observed for patients with Pi*MM COPD (any local AE: 120/150 participants [80%]; any systemic AE: 88/150 participants [57%]) or non-lung disease controls (any local AE: 116/140 participants [83%]; any systemic AE: 89/140 participants [64%]). Incidence rates for individual local and systemic AEs after the first and second doses of ChAdOx1 nCoV-19 were comparable and were numerically similar between groups (Table 2). Furthermore, severe AATD was not associated with increased AE duration for either dose, with nearly identical proportions of each study group having persistent symptoms beyond 3 and 5 days. The most frequent local AE in Pi*ZZ individuals was arm tenderness, while the most common systemic AEs were headache, fatigue, and chills/rigors. Across all 3groups, AEs were more frequently observed after the first dose of ChAdOx1 nCoV-19 than following the second vaccine dose, consistent with prior randomized controlled trials. The relative risks of individual COVID-19 vaccine-associated events in Pi*ZZ participants compared to controls are detailed in Tables S4 and S5 in the online supplement.
AEs deemed by the World Health Organization to be of special interest (AESIs) did occur but were uncommon: one Pi*ZZ participant collapsed in the context of fever the morning after their first ChAdOx1 nCoV-19 dose, 2 experienced a skin rash, and 3 demonstrated unprovoked bruising (images are provided by way of example, with consent, in Figure S2 in the online supplement). No vaccine-associated venous thromboembolism or VITT events were recorded. A complete list of AESIs is available in Table S6.
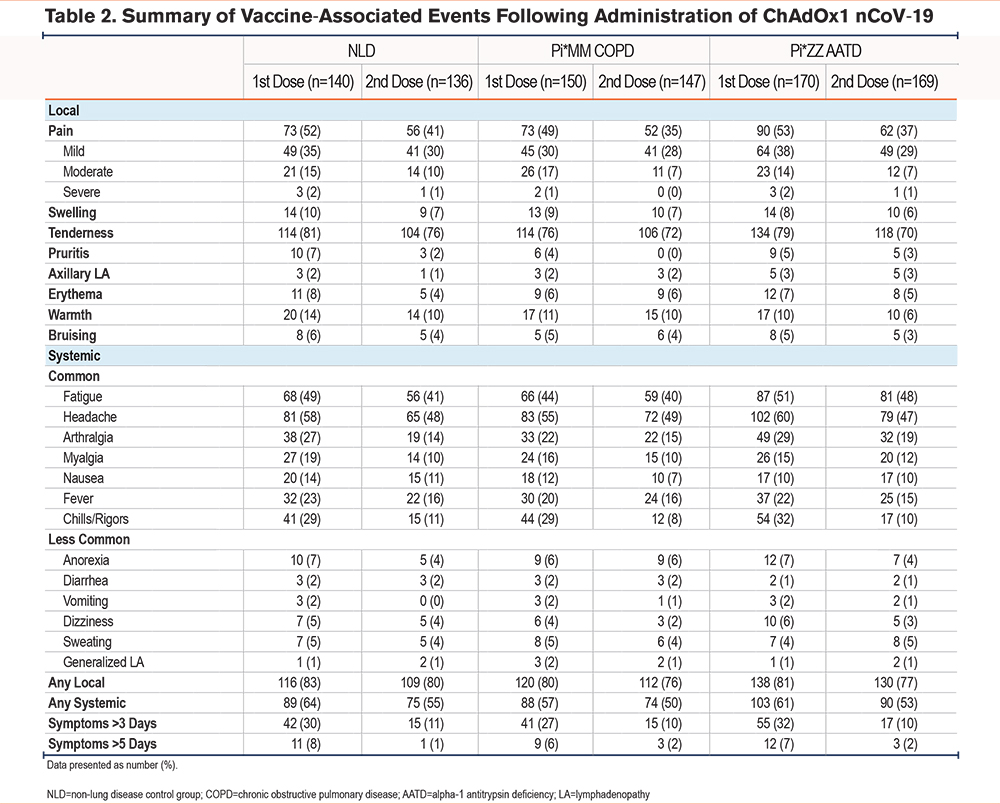
Factors Associated With Systemic and Prolonged Adverse Events in Pi*ZZ Alpha-1 Antitrypsin Deficiency Participants
Multivariate logistic regression was used to assess the association between factors hypothesized to influence the risk of postvaccine AEs and the incidence of systemic and prolonged AEs (>3 days) in Pi*ZZ individuals. Systemic AEs were associated with age ≤50 years (odds ratio [OR] 3.38, 95% confidence interval [CI] [1.48 to 7.73], Figure 1A) and female sex (2.20, 95% CI [1.14 to 4.27], Figure 1A). No association was observed between systemic AEs and IV augmentation therapy or airway obstruction. This pattern was also observed for prolonged AEs in Pi*ZZ participants (age ≤50 years: OR 2.55, 95% CI [1.07 to 6.36]; female sex: OR 2.87, 95% CI [1.43 to 5.77], Figure 1B). Similarly, age and female sex were both associated with systemic and prolonged AEs across the overall cohort; obstructive spirometry and active smoking or vaping were not (Figures 1C, 1D). It should be noted, however, that there were no participants under the age of 50 in the Pi*MM COPD group, therefore, the association between age and clinical events was primarily driven by the Pi*ZZ and non-lung disease groups. Among Pi*ZZ participants and the cohort as a whole, prevaccine safety concerns were neither associated with systemic nor prolonged AEs (Figures 1A–1D).
Self-Directed Adverse Event Reporting
At the time the study was conducted, Irish citizens receiving their initial COVID-19 vaccine series were encouraged to report serious, unusual, or persistent AEs to specialized personnel appointed by the Health Service Executive of Ireland. Of the AESIs identified, only one had been self-reported to designated Irish health officials prior to contact with the study team. Following the first dose of ChAdOx1 nCoV-19, less than 3% of systemic AEs and less than 6% of severe or prolonged AEs were successfully reported by study participants (Table S7 in the online supplement), highlighting an obstacle to accurate safety reporting at a public health level.
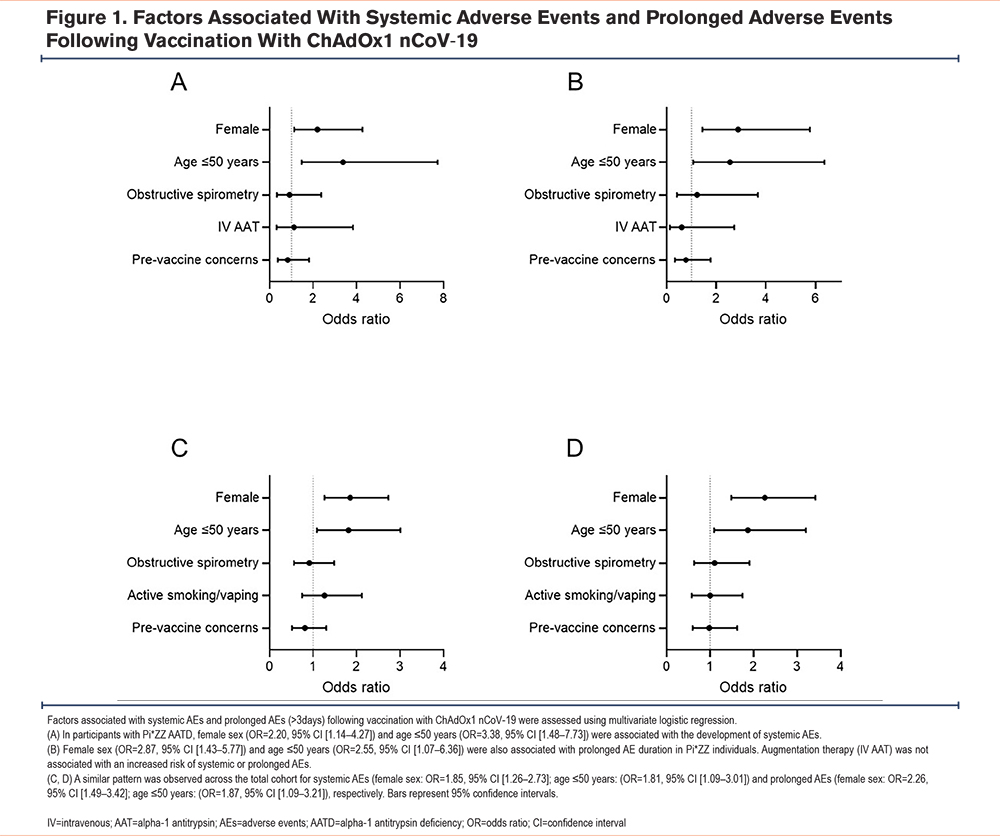
Booster Vaccination with BNT162b2 in Pi*ZZ Individuals Previously Vaccinated With ChAdOx1 nCoV-19
We next investigated the safety of mRNA-based monovalent booster vaccination with BNT162b2 in Pi*ZZ participants. Of the original 170 patients studied, 161 attended for booster vaccination. The mean interval between a second dose of ChAdOx1 nCoV-19 and BNT162b2 inoculation was 276 ± 8 days or approximately 9 months. AE rates following BNT162b2 were lower than for either dose of the initial vaccine series (Table 3). As for ChAdOx1 nCoV-19, AEs were generally mild and self-limiting; over 90% of the AEs reported were classified as Grade I or II, with the remainder Grade III, suggesting a favorable risk/benefit ratio for booster vaccination and supporting the safety of mRNA delivery systems in patients with severe AATD.
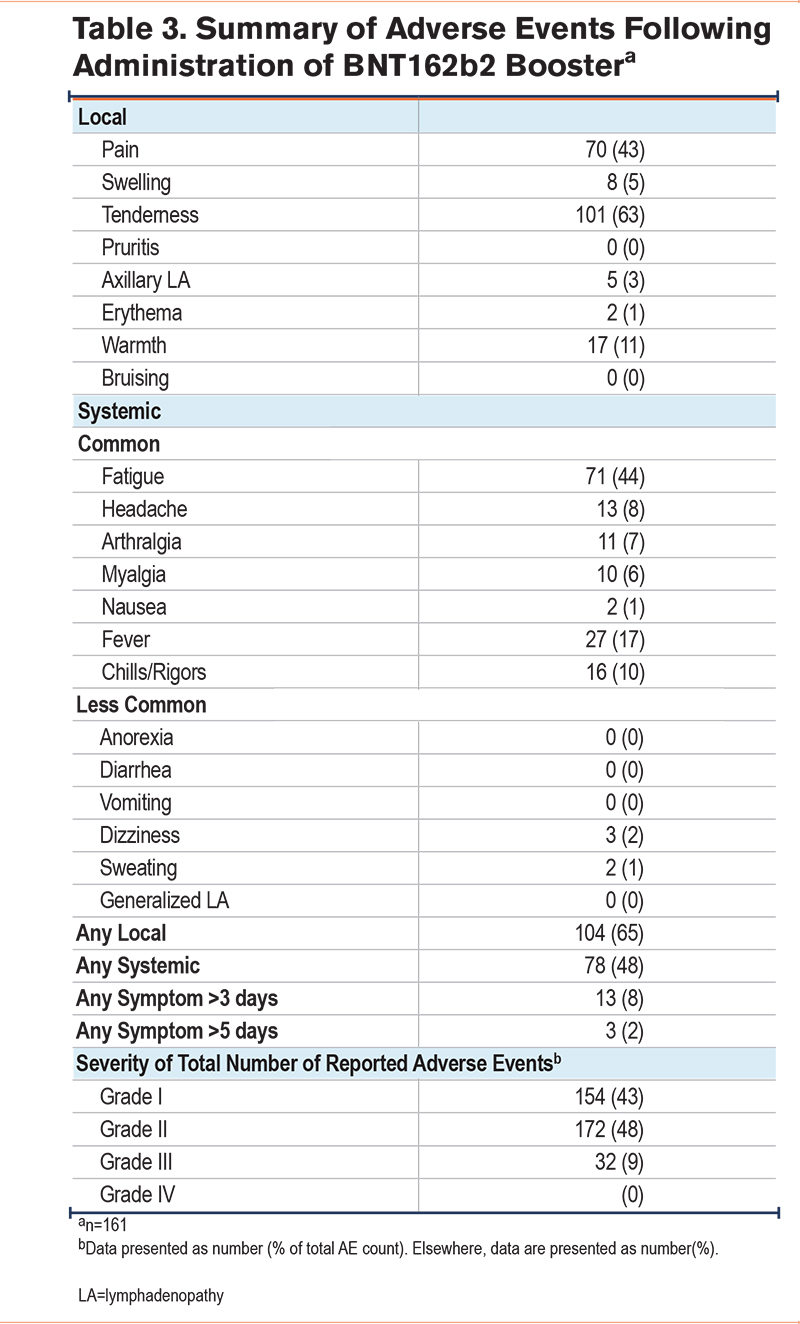
Second Booster Vaccination and Covaccination Against Influenza in Pi*ZZ Alpha-1 Antitrypsin Deficiency Participants
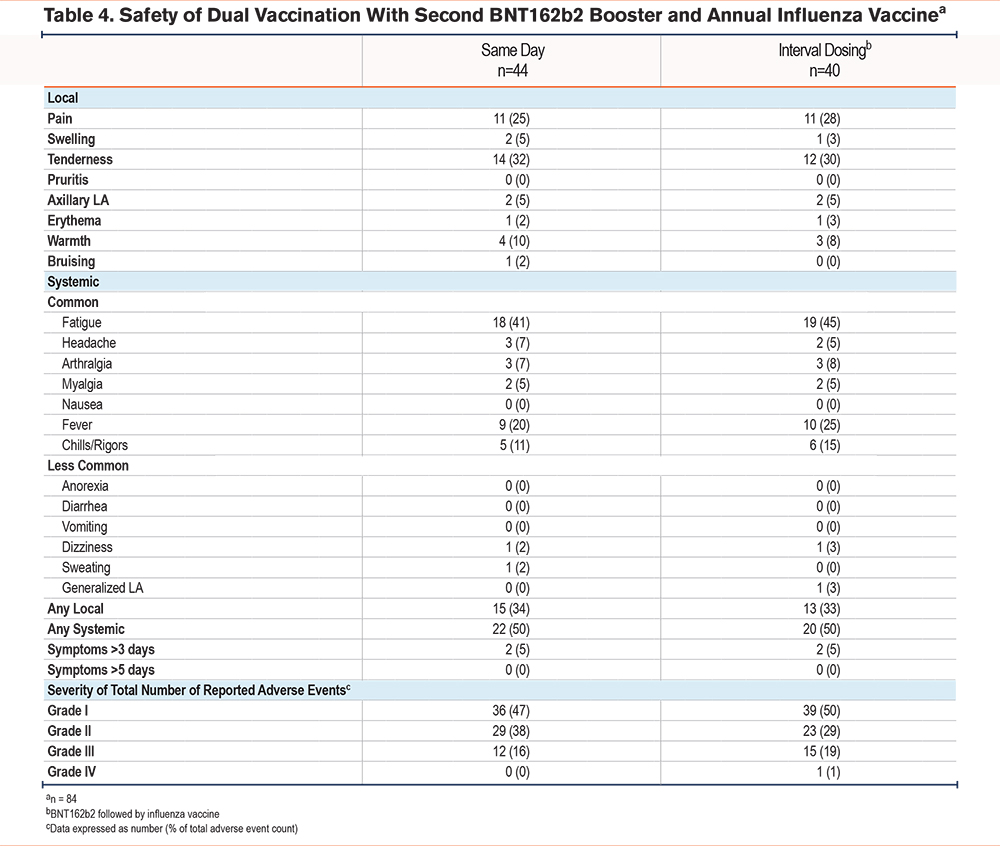
Finally, given the potential requirement for recurrent vaccination against SARS-CoV-2, we examined the safety and tolerability profiles of the bivalent COVID-19 booster vaccine in combination with the annual quadrivalent influenza vaccine in patients with Pi*ZZ AATD. Patients (n=84) were offered the option of either: (1) BNT162b2 plus the influenza vaccine in the same arm on the same day, or (2) BNT162b2 followed by the influenza vaccine 1 week later. No difference in AE incidence, duration, or severity was observed between the same-day (n=44) and interval dosing (n=40) groups, indicating that dual vaccination strategies are safe and feasible in AATD (Table 4). Fatigue, arm tenderness, arm pain, and fever were again the most frequently experienced AEs, with their incidence in both groups similar to that observed for the monovalent booster.
Discussion
Here we report the incidence and nature of vaccine-associated side-effects in people with Pi*ZZ AATD receiving sequential adenoviral vector COVID-19 vaccines, monovalent and bivalent mRNA-based booster vaccines, and annual vaccination against influenza.
Following vaccination with ChAdOx1 nCoV-19, the incidence of AEs among Pi*ZZ, Pi*MM COPD, and non-lung disease participants, for each group individually and the 3 groups collectively, was higher than in previous studies. Patients with AATD did not display increased AEs compared to Pi*MM COPD or Pi*MM non-lung disease controls, and the majority of AEs experienced across the 3 groups were relatively mild and self-limiting. Young females were the demographic at highest risk for developing systemic AEs, multiple systemic AEs, and prolonged AEs and this held true for AATD and the total population studied. Monovalent and bivalent mRNA-based boosters were well tolerated. Same-day coadministration of the latter with the annual influenza vaccine appeared feasible and equally safe compared to interval dosing.
The favorable AE profiles exhibited by ChAdOx1 nCoV-19 and BNT162b2 are relevant beyond COVID-19. Adenoviral vectors remain a candidate vehicle for gene-based therapies in AATD, and mRNA-based therapies designed to alter the functionality of the Z protein are due to enter phase 3 studies soon. Both approaches confer a risk of systemic inflammation. Our data suggest that although these technologies are likely to trigger an inflammatory response in Pi*ZZ patients, this response is unlikely to result in clinically meaningful harm.
The incidence of AEs reported here is noticeably higher than in other recent real-world studies.35-37 There are several potential reasons for this. Our study collected AE data prospectively and involved a smaller number of patients, which made intensive follow-up with detailed history-taking feasible. In contrast, studies that rely on participants voluntarily recording AEs via an app or diary-based tools may risk under-reporting milder AEs, especially when multiple AEs are present or in situations where there is participant uncertainty as to what constitutes an AE. They also confine the population studied to individuals who are sufficiently self-directed to input data accurately and on schedule. Indeed, the data described in the present study more closely approximate the results of the original placebo-controlled COVID-19 vaccine trials. The approach to capturing and characterizing AEs described here may also have implications for the frequency of patient follow-up in interventional clinical trials in AATD, where drug effects on both symptom-based and event-based exacerbations have traditionally proven elusive. Specifically, the reliability of patient-reported outcome measurements in such studies is likely to be improved by increased contact with study personnel (be that in person, via telephone, or through app-based prompts) and regular quality control.
This study is not without limitations. When studying the original first- and second-dose vaccine series, we did not perform head-to-head comparisons between the 3 cohorts described and matched cohorts receiving mRNA vaccines. This is primarily because at the time the study was conducted, mRNA vaccines were reserved for people who were over the age of 80 years, immunosuppressed, or living in residential care facilities, whereas, Irish patients with conditions such as AATD were allocated ChAdOx1 nCoV-19 only. Indeed, the mean difference in age between the Pi*ZZ and Pi*MM COPD groups studied here is slightly smaller than would be expected given their similar mean FEV1 values. Had Pi*MM COPD patients ≥80 years been eligible for inclusion, the mean age of the Pi*MM COPD group would likely have risen. When designing the study, we sought to minimize the potential confounding effects of differences in environmental factors, such as media coverage and public awareness regarding vaccine side effects, by matching the groups studied for the date of vaccination. We avoided comparisons with AATD patients vaccinated using mRNA vaccines in the months that followed since AE reporting by mRNA vaccine recipients who were dosed later would potentially be influenced by new information on vaccine safety that became available in the interim. With this in mind, it is unreasonable to conclude that mRNA vaccines such as BNT162b2 are less reactogenic than adenoviral vector vaccines based solely on the differences in postinoculation AE rates described by our data.
Assessment of vaccines in different patient populations is essential, as individuals with certain conditions may develop safety or tolerability issues more readily, and since, in the event that one vaccine was found to be unsafe in a specific disease such as AATD, alternative vaccines are typically available. Moreover, if clinicians and policymakers are to achieve buy-in from individuals who are vaccine-hesitant, a genuine and transparent approach to the evaluation and reporting of real-world patient safety data is required. This study is smaller than similar studies in other chronic conditions such as diabetes mellitus or rheumatoid arthritis. However, it is considerably larger than most prospective observational studies of Pi*ZZ AATD published to date, involves intensive follow-up of symptoms, and represents the first report of vaccine safety and reactogenicity – COVID-19, influenza, or otherwise – focused on this rare disease.
Taken together, these results suggest that COVID-19 vaccination in patients with severe AATD, is not associated with a greater risk of developing AEs than that observed in the general population, further refuting speculation that vaccination may be unsafe in patients with pre-existing chronic inflammatory lung diseases. Based on these data, COVID-19 vaccines should, therefore, continue to be offered to Pi*ZZ individuals, including those with advanced lung disease.
Acknowledgements
Author contributions: OJMcE, TPC, CHG, and NGMcE conceptualized the study. OJMcE, DDF, GK, BC, DP, EO’C, PB, CG, and TPC recruited patients and collected data. OJMcE, BC, OFMcE, DDF, MPM, TPC, CHG, and NGMcE analyzed data. OJMcE, TPC, CHG, and NGMcE wrote the manuscript. All authors reviewed and edited the manuscript prior to submission.
The authors wish to acknowledge the study participants and their families, Prof. Gillian Vale for guidance regarding ethical design and data protection, and the health care staff involved in coordinating vaccine clinics involving study participants, in particular the nursing staff at Beaumont Hospital, Dublin.
Declaration of Interests
OJMcE has been an investigator for Chiesi and Grifols. CHG has been an investigator for Boehringer Ingelheim and a drug safety monitoring board chair for Novartis. NGMcE has been an investigator in clinical trials for CSL Behring, Galapagos, Chiesi, and Vertex, and reports personal fees, all outside the present unfunded work, from CSL Behring, Grifols, Chiesi, and Shire. The remaining authors have no conflicts to disclose.