Running Head: Inflammation, Lung Function, and Physical Activity
Funding Support: CASCADE was funded in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI) R01HL093146 and UL1RR025014. This work was also supported by NHLBI R01HL140971-01A1 and with resources and the use of facilities at the Minneapolis VA Health Care System, Minneapolis, Minnesota.
Date of Acceptance: May 31, 2024 | Publication Online Date: June 5, 2024
Abbreviations: BMI=body mass index; CASCADE=COPD Activity: Serotonin Transporter, Cytokines, and Depression study; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRP=C-reactive protein; ECLIPSE=Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints study; FEV1=forced expiratory volume in 1 second; FEV1%pred=forced expiratory volume in 1 second percentage predicted; ICS=inhaled corticosteroid; IL=interleukin; IL-6=interleukin-6; OFDR=overall false discovery rate; SD=standard deviation; TNF-α=tumor necrosis factor
Citation: MacDonald DM, Samorodnitsky S, Lock EF, et al. Biomarkers of inflammation and longitudinal evaluation of lung function, physical activity, and grip strength: a secondary analysis in the CASCADE study. Chronic Obstr Pulm Dis. 2024; 11(4): 396-405. doi: http://doi.org/10.15326/jcopdf.2024.0500
Online Supplemental Material: Read Online Supplemental Material (520KB)
Note: Portions of these data were presented as a poster at the American Thoracic Society 2023 International Conference, Washington, DC.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by fixed airflow obstruction on spirometry, a history of exposure to noxious stimuli such as cigarette smoke, and chronic respiratory symptoms.1 COPD is associated with chronic lung and systemic inflammation, and once the inflammatory process is established, it often persists despite smoking cessation or removal of other inciting factors.2 Lung function decline and respiratory symptoms similarly can progress despite the removal of noxious stimuli and faster lung function decline is also associated with increased mortality and COPD hospitalizations.3,4 Despite these well-established associations, attempts to identify inflammatory biomarkers that predict lung function decline have been unsuccessful.5
Another important prognostic marker in COPD is physical activity. Reduced physical activity is common in people with COPD and is associated with faster lung function decline, increased rates of COPD hospitalizations and rehospitalizations, and higher mortality.6-9 Associations between systemic inflammation and physical activity among people with COPD are complex. At least one study found that higher physical activity is associated with higher systemic inflammation,10 while others have found that decreased physical activity and hand grip strength are associated with higher systemic inflammation.11-14 Exercise interventions in COPD have been shown to decrease systemic inflammation.15 Exercise-inflammation relationships may also be influenced by body mass index, malnutrition, and duration between the end of physical activity and measurement of inflammation.16,17
Reducing lung function decline and increasing physical activity are important targets in people with COPD, but interventions thus far have been only modestly successful. With an objective to identify potential etiologic and therapeutic pathways, we tested the hypothesis that higher systemic inflammation is associated with faster lung function decline and reductions in physical activity and strength.
Methods
A full description of the statistical analysis methods is included in the online supplement.
Participants
The COPD Activity: Serotonin Transporter, Cytokines, and Depression (CASCADE) study (NCT01074515) was an observational study of participants with COPD to study the biologic effects and functional consequences of depression in people with COPD.18-20 Full inclusion and exclusion criteria have been published previously,19 but briefly, inclusion criteria were: (1) a diagnosis of COPD based upon postbronchodilator spirometry (forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio <0.7 and FEV1 <80% predicted); (2) greater than 10 pack years of current or past cigarette smoking; (3) no acute exacerbation of COPD in the last 4 weeks, and (4) the ability to speak, read, and write in English. Because CASCADE was a study of inflammation, those with other conditions that could cause changes in inflammation were excluded. These conditions included other chronic lung diseases, chronic inflammatory diseases, uncompensated heart failure, autoimmune disease, lung or metastatic cancer, and chronic oral prednisone use. Patients who were unable to walk and could not complete the 6-minute walk test were also excluded. We included all participants with baseline biomarker measurements in this analysis.
Procedures
Institutional review boards at each site approved the study and written informed consent was obtained from each participant. Spirometry was performed according to American Thoracic Society standards using an EasyOne spirometry (ndd Medical Technologies Inc, Andover, Massachusetts), and postbronchodilator spirometry was used in this analysis. A 6-minute walk test was performed to determine the total distance walked. Physical activity was measured with a Stepwatch 3 Activity Monitor fastened above the right ankle. Participants were asked to wear this for 7 days during waking hours. A valid day was defined as having ≥ 600 minutes of monitor wear time. Grip strength was measured using a hand dynamometer on the dominant hand. The participant was asked to hold the dynamometer at a 90-degree angle from the body and squeeze the grip handle as hard as they could. The measurement was repeated 3 times, and the best result was used. Spirometry, physical activity monitoring, and grip strength were performed at baseline and repeated at the year 1 and year 2 follow-up visits.
A full description of biomarker procedures has been previously published.20 Plasma biomarkers included C-reactive protein (CRP) measured using a duoset Enzyme-Linked Immunosorbent Assay (R&D Systems, Minneapolis, Minnesota) and interleukin (IL)-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, interferon, granulocyte macrophage-colony stimulating factor, and tumor necrosis factor (TNF-α) measured on a Luminex multiplex platform. These 14 biomarkers were chosen for CASCADE based on previous association with COPD and/or depression.20-23
Statistical Analysis
Biomarkers levels were transformed using a log(1+x) transformation. After transformation, all biomarkers were centered and scaled to mean 0, standard deviation 1.
The outcomes were: FEV1, total distance walked, average steps total, average minutes active, average percentage time spent inactive, and grip strength. We measured changes in outcomes as the raw change: (outcomeyear2 – outcomebaseline), and as the percentage change: (outcomeyear2 – outcomebaseline)/outcomebaseline.
We first implemented an unadjusted analysis for the associations between baseline biomarkers and patient outcomes. We calculated the Pearson correlation between each baseline biomarker and change in each outcome (raw and percentage change) and tested the null hypothesis that this correlation is equal to 0. To control the overall false discovery rate (OFDR) across the biomarkers, we used a hierarchical hypothesis testing procedure to test the significance of the correlation between each biomarker and each outcome.24 We treated each biomarker as a “set” of hypotheses in which 12 hypotheses (raw and percentage change in 6 outcomes) were tested. The null hypotheses were that a given biomarker is not associated with raw or percentage change in the outcomes. By controlling the OFDR, we controlled the expected proportion of biomarker sets falsely rejected. We report if we could reject any hypotheses within a biomarker set at the OFDR<0.05 level, and if so, which hypotheses within that set that were rejected and the resulting Pearson correlations.
We also used an adjusted linear mixed modeling analysis to investigate the relationship between baseline biomarkers and the outcomes of interest over time (testing associations between baseline biomarkers and outcomes at baseline, year 1, and year 2 rather than changes in outcomes). We applied the same hierarchical hypothesis testing framework for each biomarker to assess significance. We adjusted for baseline biomarker, time, and smoking status at the year of follow-up as fixed effects and adjusted for FEV1 in the models for non-FEV1 outcomes. We included an interaction between baseline biomarker and time to capture any change over time of the baseline biomarker’s effect on the outcome and included a random intercept for each participant to account for participant-specific variation in the observations.
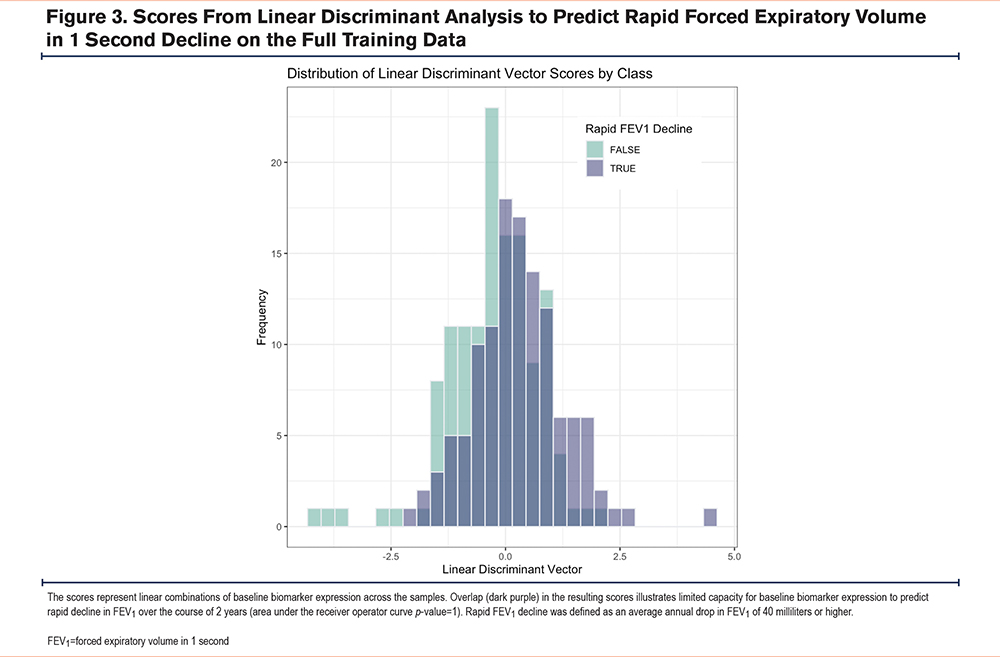
Finally, in addition to testing associations between individual baseline biomarkers and changes in FEV1, we also tested if linear combinations of baseline biomarkers predict rapid FEV1 decline from baseline to year 2 using Fisher’s linear discriminant analysis. We defined rapid FEV1 decline as an average annual drop in FEV1 of 40 milliliters or higher. We used the area under the receiver operating curve under 10-fold cross-validation to evaluate the predictive performance, and a permutation testing approach to assess significance.
Results
Baseline characteristics for all participants (Total), and for participants stratified by rapid FEV1 decline are shown in Table 1. Baseline levels of inflammation have been previously published.20 Participants had a mean (standard deviation) age of 67.5 (8.5) years, 19.5% were female, and 28.5% were currently smoking. Mean FEV1 percentage predicted was 45.0 (15.8) at baseline and 188 (62.3%) participants were on inhaled corticosteroids. Average annual changes in FEV1, average minutes active, average steps total, 6-minute walk distance, percentage time spent inactive, and grip strength are included in Supplemental Table S1 in the online supplement.
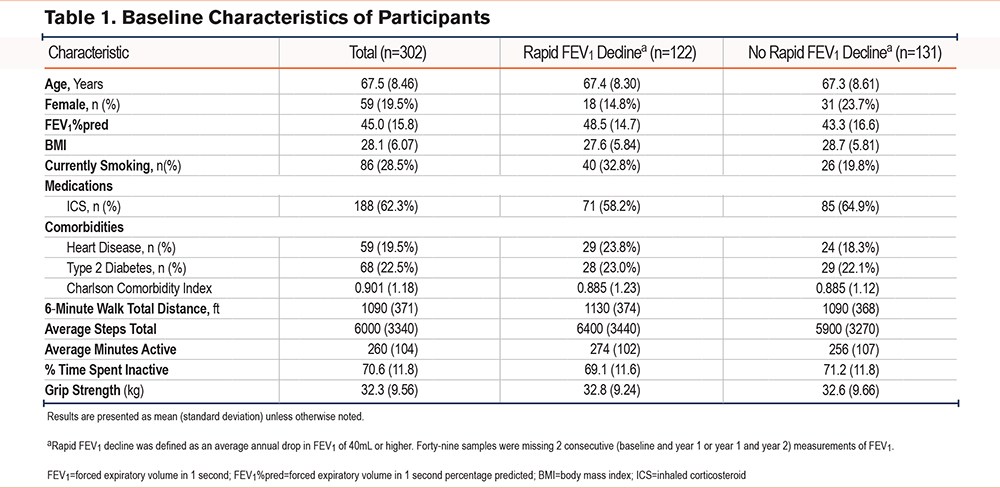
Baseline Biomarkers and Longitudinal Changes in Physical Activity and Grip Strength (Unadjusted)
The screening hypothesis was rejected for only 1 biomarker/outcome pair testing associations between baseline biomarkers and longitudinal changes in activity and grip strength. Higher IL-6 was associated with a greater percentage change in average minutes active (Figure 1a), but after removal of 2 outliers, this relationship was attenuated (Figure 1b).
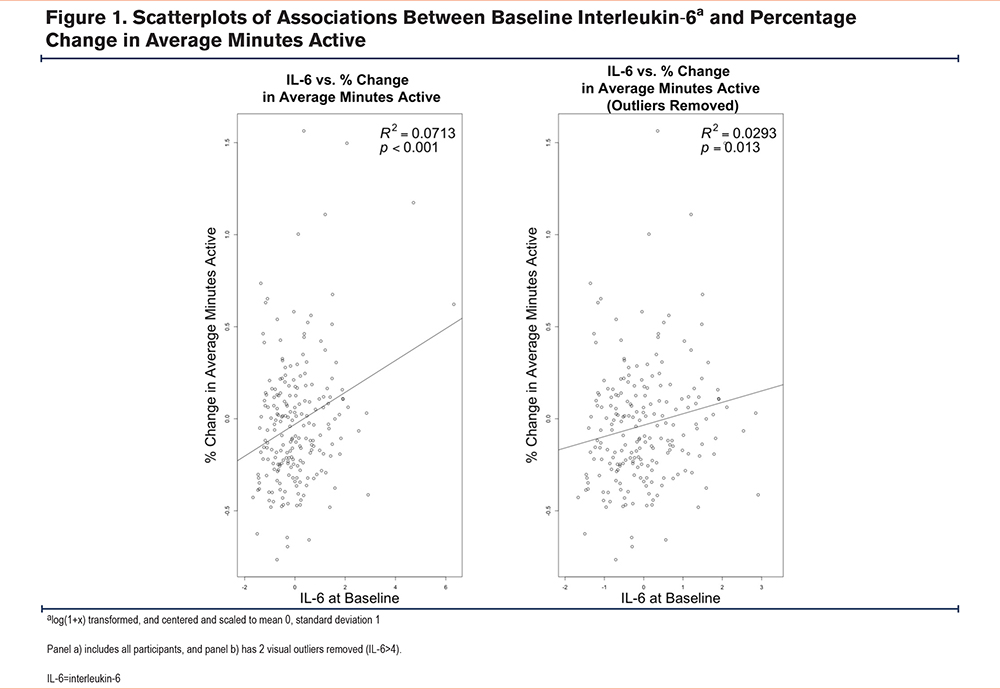
Baseline Biomarkers and Associations With Physical Activity and Grip Strength at Baseline, Year 1, and Year 2 (Unadjusted and Adjusted)
In our linear mixed modeling analysis (adjusted for smoking, time, baseline biomarker, and FEV1), we rejected 2 hypotheses for IL-6 and 4 hypotheses for CRP.
In unadjusted and adjusted analyses, higher baseline IL-6 was associated with lower average minutes active and higher average percentage time inactive at baseline and year 1, but results were attenuated at year 2 (unadjusted analyses: Figure 2, adjusted analyses: Table 2).
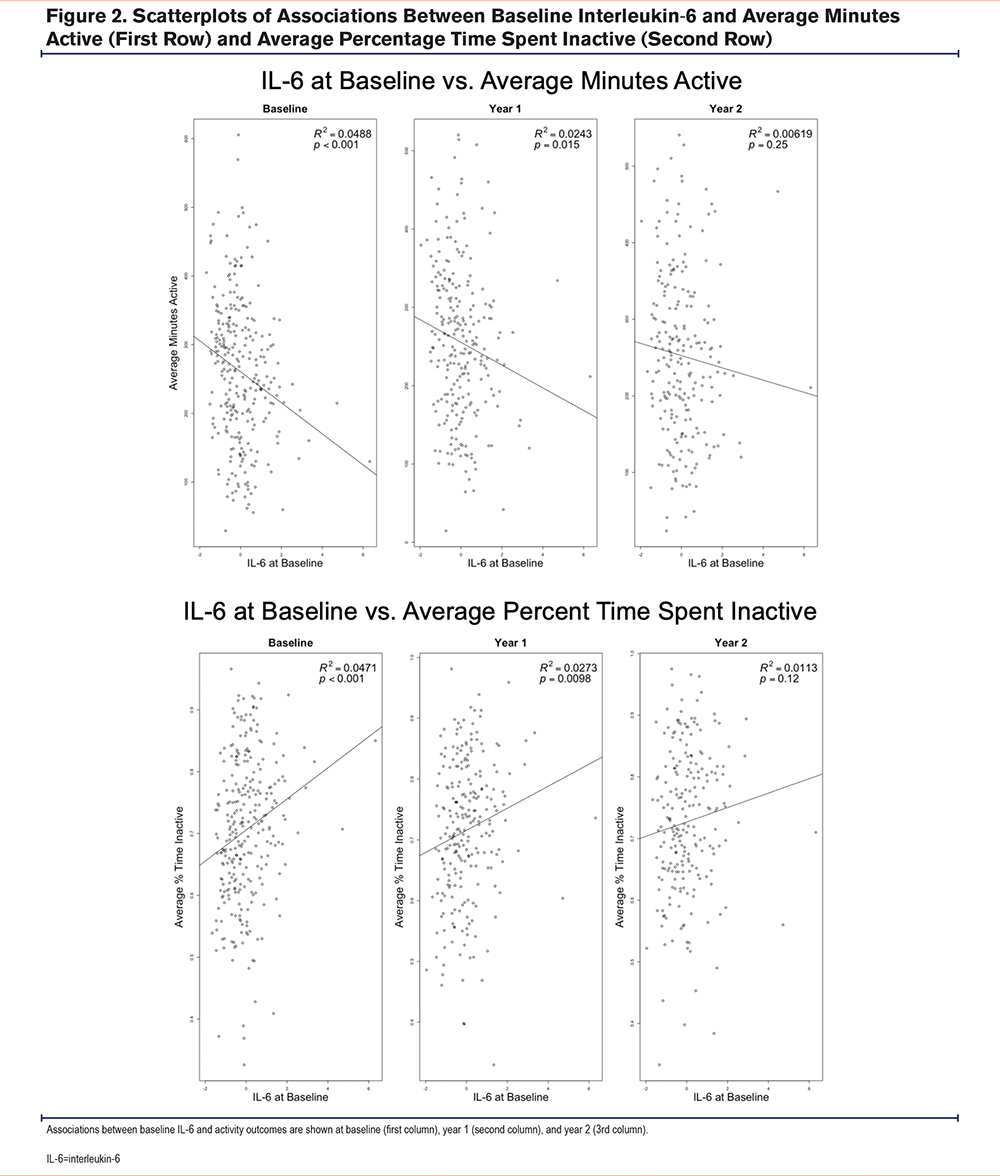
In unadjusted and adjusted analyses, higher baseline CRP was associated with lower total distance walked, lower average minutes active, lower average steps total, and higher average percentage time spent inactive at baseline, year 1, and year 2 (unadjusted analyses: Supplemental Figure 1 in the online supplement, adjusted analyses: Table 2).
Baseline Biomarkers and Forced Expiratory Volume in 1 Second
The screening hypotheses were not rejected for absolute or percentage change in FEV1, or for associations between baseline biomarkers and FEV1 over time (at baseline, year 1, and year 2).
Results from linear discriminant analysis, in which we examined if linear combinations of baseline biomarkers predicted rapid FEV1 decline are shown in Figure 3. The p-value for the area under the receiver operator curve was 1, implying that the predictive performance was not significantly better than random guessing.
Discussion
In this analysis of associations between inflammatory biomarkers and lung function, grip strength, and physical activity we found no strong associations between inflammation and longitudinal changes in these physiologic outcomes.
Our primary goal was to test associations between baseline biomarkers and longitudinal change in lung function. Systemic inflammation has been proposed as a driver of lung function decline in general, smoking-related COPD and in other causes for COPD, such as HIV. We found no association between inflammatory biomarkers and several measures of FEV1 decline. This is consistent with prior studies, most notably the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study which tested associations between baseline biomarkers and longitudinal changes in FEV1 in 1793 participants.5 In ECLIPSE, IL-8 and CRP were associated with baseline FEV1, but not with longitudinal changes in FEV1. Higher levels of the pneumoprotein club cell secretory protein were associated with slower decline in FEV1 in ECLIPSE, but pneumoproteins were not measured in CASCADE. ECLIPSE analyzed a total of 5 inflammatory biomarkers (CRP, IL-6, IL-8, TNF-α, and fibrinogen) and found that 4 of these (CRP, IL-6, IL-8, and TNF-α) were not associated with longitudinal lung function decline. We analyzed an additional 8 biomarkers not analyzed in ECLIPSE that included IL-4 and IL-13 which are involved in allergic inflammation, and IL-10, a key anti-inflammatory mediator.25,26 Our results in this study are also consistent with our prior results analyzing associations between systemic inflammation and lung function decline in people living with HIV, where we found that higher inflammatory biomarkers (including IL-6 and CRP) were not associated with faster lung function decline.27,28 Our data provide further evidence that inflammatory biomarkers measured in the blood do not predict lung function decline and suggest that alternative sources for markers of lung function decline such as bronchoalveolar lavage fluid29 or physiologic/imaging30 measurements should be pursued.
We also tested associations between inflammatory biomarkers and physical activity outcomes. Like our lung function analysis, we found no associations between baseline inflammatory biomarkers and longitudinal changes in physical activity or strength. We did find that higher concentrations of inflammatory biomarkers were associated with lower physical activity at baseline and that association persisted over time, with higher systemic inflammation predicting lower physical activity up to 2 years later. Prior studies analyzing the randomized effects of exercise on systemic inflammation found that physical activity leads to decreased systemic inflammation.15 Physical activity may also be particularly beneficial in people with COPD with increased systemic inflammation. In a study of 385 participants with COPD, physical activity was not associated with all-cause mortality among all participants, or in those without elevated CRP; however, in those with an elevated CRP, each 60-minute increase in physical activity was associated with a 31% reduction (hazard ratio 0.69, 95% confidence interval 0.51 to 0.93) in all-cause mortality over 78 months of follow-up.31 These findings suggest that it is important to encourage patients with COPD to increase physical activity, and highlight the importance of interventions to improve physical activity such as pulmonary rehabilitation. Associations between inflammatory biomarkers and activity may vary around the time of an acute exacerbation of COPD. For example, among 50 people with COPD experiencing an acute exacerbation, higher CRP was associated with a greater decrease in 6-minute walk distance at day 3 of the exacerbation.32 We did not have biomarker measurements at the time of exacerbation and were not able to test if higher inflammatory biomarker concentrations at the time of exacerbation led to longitudinal changes in activity.
Our study has several limitations. Previous studies that found an association between baseline biomarkers and longitudinal changes in outcomes were much larger, and we were underpowered to detect small effects that can be seen in biomarker studies. The small sample size also limited our ability to test associations between biomarkers and clinically important outcomes such as exacerbations or mortality. Metabolites and biomarkers present in lung samples are not necessarily found in peripheral blood samples,29 and CASCADE did not collect samples from other sites such as bronchoalveolar lavage fluid or perform chest imaging and we were unable to test if these other potentially important markers predicted lung function or physical activity changes.
Our study also has several strengths. We were able to test associations between baseline biomarkers and longitudinal changes in a variety of important physiologic outcomes in more than 300 well-characterized participants over a period of 2 years. We also used a hierarchical hypothesis testing framework that limited the overall false discovery rate (the rate that biomarkers deemed significant are truly null), an important consideration in studies testing multiple biomarkers.
In conclusion, baseline biomarkers of inflammation were not associated with longitudinal changes in lung function, physical activity, or grip strength. These data provide further evidence that systemic inflammation does not drive lung function decline in COPD, and do not support targeting systemic inflammation for improvement of lung function, physical activity, or strength. Future studies should investigate noninflammatory pathways and alternative samples such as bronchoalveolar lavage fluid.
Acknowledgements
Author contributions: DMM, CWH, and VF conceived the study. DMM, CWH, VF, EFL, and SS designed the study. VF and HQN obtained funding. PL and HQN acquired the data. EFL and SS performed the primary statistical analysis. DMM drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the views of the United States Government, the Department of Veterans Affairs, the National Heart, Lung, and Blood Institute, or any of the authors’ affiliated academic institutions.
We would like to thank Dr. Gustavo Matute-Belle for his assistance with measuring the biomarker concentrations for this project. All work contributed by Dr. Matute-Bello was done prior to his employment by the National Heart, Lung, and Blood Institute.
Declaration of Interests
The authors have no conflicts of interest to declare.