Running Head: Digihaler in COPD
Funding Support: This study was funded by Teva Branded Pharmaceutical Products Research and Development, Inc.
Date of Acceptance: May 9, 2025 | Publication Online Date: May 27, 2025
Abbreviations: AECOPDs=acute exacerbations of chronic obstructive pulmonary disease; Post-BD=postbronchodilator; BMI=body mass index; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; ICS=inhaled corticosteroid; LABA=long-acting beta2-agonist; LAMA=long-acting muscarinic antagonist; mMRC=modified Medical Research Council; PIF=peak inspiratory flow; SABA=short-acting beta2-agonist; SD=standard deviation; SE=standard error
Citation: Drummond MB, Hemphill CC, Hill T, Boe A, Yu D, Ohar JA. Use of a digital inhaler to assess COPD disease variability and identify impending acute COPD exacerbations: a pilot study. Chronic Obstr Pulm Dis. 2025; 12(3): 250-259. doi: http://doi.org/10.15326/jcopdf.2024.0555
Online Supplemental Material: Read Online Supplemental Material (342KB)
Note: Abstracts related to this study were presented in poster form at the American Thoracic Society 2023 International Conference.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disorder characterized by the presence of intermittent flares or exacerbations.1 Thirty-four percent of newly diagnosed COPD patients will experience at least one acute exacerbation of COPD (AECOPD) within the first year, increasing to 52% within the first 4 years of diagnosis.2 AECOPD is associated with accelerated loss of lung function, reduced quality of life, decreases in exercise performance, and mortality.3,4 Frequent exacerbators experience more profound and prolonged deficits in lung function, quality of life, and physical function.3-6
AECOPDs tend to be cyclic in nature, with increased risk for a subsequent AECOPD within the first 8 weeks of the last exacerbation.7 The time interval between AECOPDs tends to shorten, leading to repeated hospitalizations that culminate in death, with 25% of hospitalized patients dead in a year and 65% dead within 5 years.7,8 AECOPD events are symptom-defined by an acute worsening of cough, sputum, or dyspnea.1 Early intervention can reduce the burden of AECOPDs by mitigating the impact on health status,9 emergency hospitalization,10 mortality,11 and accelerating the rate of recovery.9
Early signs of an AECOPD include physiologic changes such as increased respiratory and heart rates and decreased lung volumes.12 While heart and respiratory rates are easily and reliably measured by wearables, home measurement of lung volumes is more problematic.13 Accuracy of home spirometry measurements without the benefit of coaching has been disappointing.14 While some studies have used digital inhalers in COPD to monitor adherence or support correct inhaler technique, there has been limited research on the use of digital inhalers that incorporate dynamic physiologic and inhaler medication use patterns to identify impending AECOPDs.15,16
The ProAir Digihaler is a medium-high resistance digital inhaler that measures and records inhaler parameters such as peak inspiratory flow rate (PIF), inhalation volume, inhalation duration, and time to peak inhalation as well as the number of inhalations and other inhaler events (i.e., air vent block, opening and closing the inhaler with no inhalation).17,18 The ProAir Digihaler connects to a companion app to download the data for patients and clinicians to view. Data from the ProAir Digihaler device has the potential to help clinicians evaluate short-acting beta2-agonist (SABA) use as a marker of COPD disease variability and can be used to identify inspiratory flow metrics that represent the onset of an AECOPD. A previous study using ProAir Digihaler without the patient-facing app in COPD patients demonstrated that inhalation parameters captured by the Digihaler declined prior to an AECOPD.19 The study presented here extends prior work by conducting a real-world pilot study to evaluate variations in the ProAir Digihaler inhaler metrics and the potential to identify impending AECOPDs using the digital inhaler data from an exacerbation-enriched COPD population. This study was unique in that patients were enrolled remotely and used a novel version of the companion app that would ask about patient symptoms in response to triggers of worsening COPD status based on their inhaler use and inhaler parameter data.
The primary objective of this pilot study was to determine the variation in ProAir Digihaler metrics (PIF, inhalation volume, number of inhalation events, inhalation duration, and time to peak inhalation) among COPD patients in the ambulatory setting. Secondary objectives included the correlation of self-reported SABA use with actual SABA use, inhaler metrics (PIF, inhalation volume, number of inhalation events) with monthly COPD Assessment Test (CAT) score domains, and the correlation of inhaler metrics with a daily self-assessment prompted by the app. An exploratory objective was to determine the change in inhaler metrics in patients experiencing an AECOPD during the study period.
Methods
Study Design
This was a phase 4, open-label, 3-month real-world observational pilot study conducted at 2 centers in the United States (University of North Carolina at Chapel Hill and Wake Forest University in Winston-Salem, North Carolina) between March 1, 2022, and February 13, 2023. The study cohort consisted of patients followed in the ambulatory setting with documented COPD (postbronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity and <0.70 within the last 2 years and an FEV1≤80% predicted) and a history of 2 moderate or one severe AECOPD in the prior 12 months, who consented to participate. The complete study inclusion and exclusion criteria are listed in Supplemental Table E1 in the online supplement.
This study was designed to be conducted entirely virtually to minimize any potential interruptions related to the COVID-19 pandemic. Participants underwent a virtual screening/enrollment visit, during which informed consent was obtained using a verbal script. Once enrolled, participants were mailed 3 ProAir Digihaler inhalers and were instructed to contact a central call center for app and dashboard onboarding. Participants were asked to use the ProAir Digihaler as their primary mode of SABA therapy as they would in usual treatment and as indicated in the product package insert and instructions for use.
Study coordinators contacted participants to ensure set-up and correct usage within 1 week of drug receipt. Throughout the study, participants had access to objective information on their inhaler use through the app, but study sites did not have access to the dashboard and did not monitor the participants’ inhaler use in real-time. The dashboard was used periodically but not in real-time by the central call center to ensure ongoing data collection from the participants’ inhalers. The modified Medical Research Council (mMRC) score was collected during the enrollment visit. Study coordinators phoned participants once per month to collect a CAT score and self-reported average albuterol use over the preceding month. Participants were also asked about any AECOPD events requiring treatment with antibiotics and/or steroids or hospitalization in the prior month. Participants experiencing an AECOPD occurring within 2 weeks of the end of the study were followed for 14 days after an AECOPD resolution. Total participant duration was 3 months (+21 days to account for 14 days in the event of an AECOPD and 7-day window at the end of the study). The study was approved by the local institutional review board at each participating site (University of North Carolina-Chapel Hill and Wake Forest University) and was registered at clinicaltrials.gov (NCT05241288).
Statistical Analysis
Given the exploratory nature of this pilot study, most endpoints were analyzed using descriptive statistics with the results reported in means with standard deviation (SD) or standard error, counts, and percentages. Patient-reported SABA use was evaluated independently on a per-month basis for comparisons with actual monthly use. Correlations between CAT and inhaler metrics were analyzed, and the correlation coefficients are reported. Statistical analyses were completed using SAS v9.4 software. The primary considerations in choosing the proposed target sample size were the unit of analysis, patient availability, fiscal costs, time required to complete the study, the proportion of participants expected to complete the protocol, and conjectures about the precision of the estimators of interest. It should be noted that the unit of analysis was not the individual patient but, instead, the number of inhalation events. Previous research using the Proair Digihaler helped to inform the potential volume of data that could be captured from a small number of patients to feasibly examine variability in the parameters in this pilot study. This study did not have prespecified power calculations for patient sample size given that this digital inhaler and novel companion app was first ever used in this study and all aforementioned considerations.
Results
Study Cohort
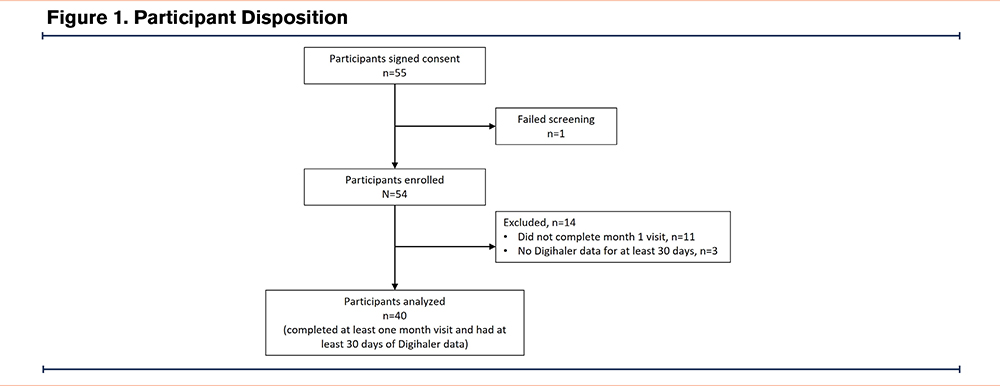
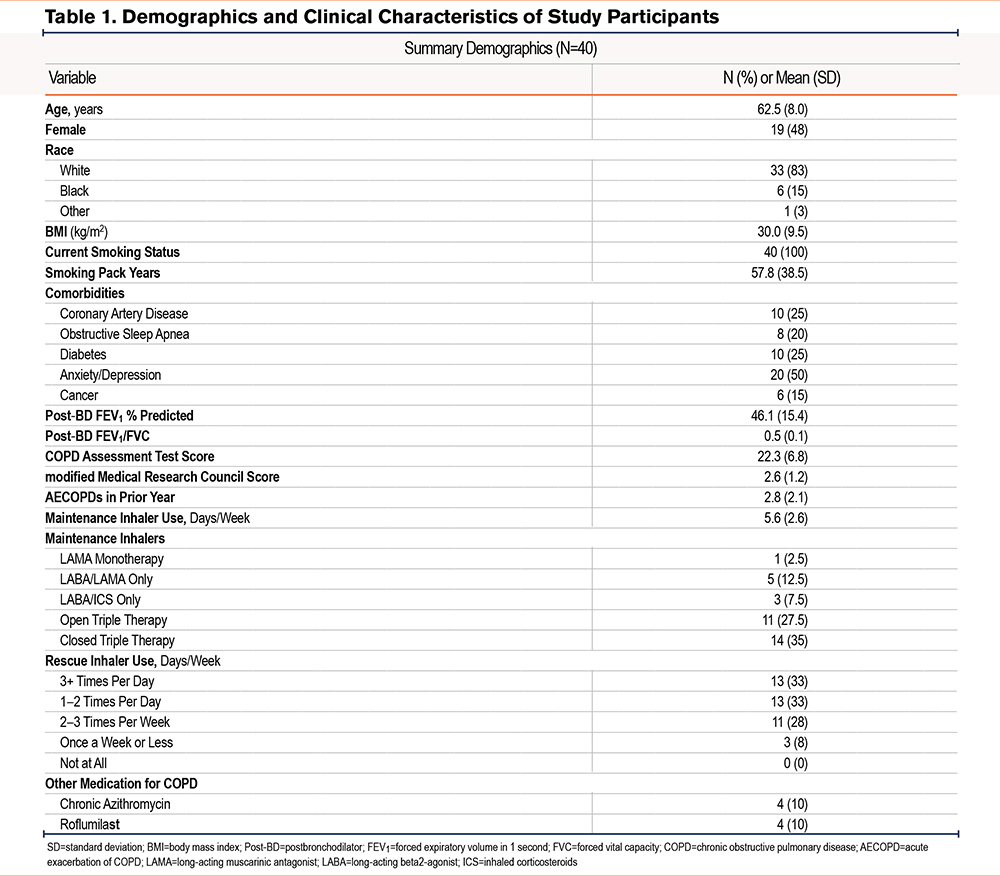
A total of 55 participants were enrolled (N=25 at UNC, N=30 at Wake Forest). Overall, 49 participants completed week 1, 43 participants completed month 1, and 39 participants completed month 2 and 3 phone visits. The analytical cohort for this analysis (n=40 patients, 9649 inhalations) included all participants who completed the month 1 phone visit and had at least 30 days of Digihaler data. The full participant disposition is shown in Figure 1. The demographic and clinical characteristics of the cohort are summarized in Table 1. Overall, the cohort was approximately 62 years old, nearly half female and the majority White race. All were current smokers, with a mean FEV1 of 46% predicted experiencing a mean (SD) of 2.8 (2.1) AECOPDs in the prior year. Comorbidities were common in the cohort, with the most prevalent comorbidity being anxiety/depression (50%). The cohort was highly symptomatic, with mean (SD) CAT scores of 22.3 (6.8), a mean (SD) mMRC of 2.63 (1.2), and with 66% of the cohort using a rescue inhaler at least once per day.
Variation in ProAir Digihaler Metrics
Over the 3-month study, data from 9649 inhalations were captured. Participants had a mean (SD) SABA use of 2.3 (2.63) inhalations per day during the study, with a wide range of daily use (0–23 inhalations per day). Across all inhalations, the mean (SD) PIF was 67.6 (20.3)L/min. A total of 39% of inhalation events had PIF<60L/min, with 1.2% of inhalation events with PIF<30L/min. The mean (SD) inhalation volume was 1.40 (0.60)L, with the range of inhalation volume 0.047 to 6.74L (Supplemental Figure E1 in the online supplement).
Correlation of Self-Reported Short-Acting Beta2-Agonist Use With Actual Short-Acting Beta2-Agonist Use
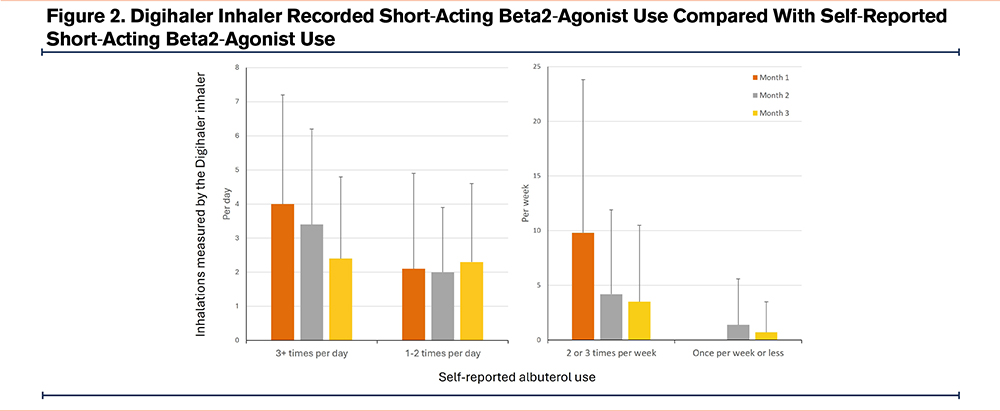
At enrollment, 8% of participants reported albuterol rescue use once a week or less, with 28% reporting use 2–3 times per week, 33% reporting use 1–2 times per day, and 33% reporting use 3 or more times per day. Monthly recall of self-reported SABA use at interim phone visits was well correlated with actual SABA use captured by the Digihaler inhaler (Figure 2 and Supplemental Table E2 in the online supplement). Specifically, participants reporting use once per week or less had actual use of 0.7 inhalations per week in month 3. Those reporting use 2–3 times per week actually used 3.5 inhalations per week in month 3. Participants reporting rescue use 1–2 times per day had an actual use of 2.3 inhalations per day in month 3, while those reporting 3 or more inhalations per day had actual use of 2.4 inhalations per day in month 3. This correlation was robust regardless of duration of enrollment in the study.
Correlation of Inhaler Metrics With Patient-Reported Outcomes
Patients had the option within the Digihaler App to respond to a daily self-assessment asking them to “select the face that best represents how you’ve been feeling today,” with responses provided on a Leikert scale in the form of facial icons (Supplemental Figure E2 in the online supplement). While most inhaler metrics were statistically significantly different between groups of responses, the directionality of changes was not consistent, and the difference in values are likely not of clinical significance (Supplemental Table E3 in the online supplement). However, the number of SABA inhalations per day was highest in the “frowning face” group, demonstrating correlation between Leikert scale responses with increased rescue albuterol use. There was poor correlation between CAT score and the average of Digihaler metrics captured in the 30 days prior to the CAT score reporting date, regardless of time in study, with correlation coefficients ranging from -0.2 to 0.1 (Supplemental Table E4 in the online supplement).
Change in Inhaler Metrics in Patients Experiencing an Acute Exacerbation of COPD
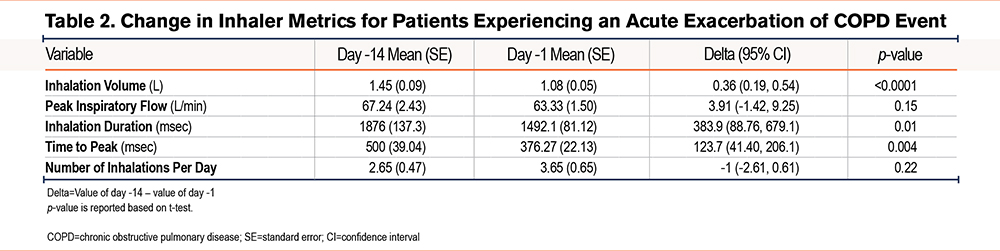
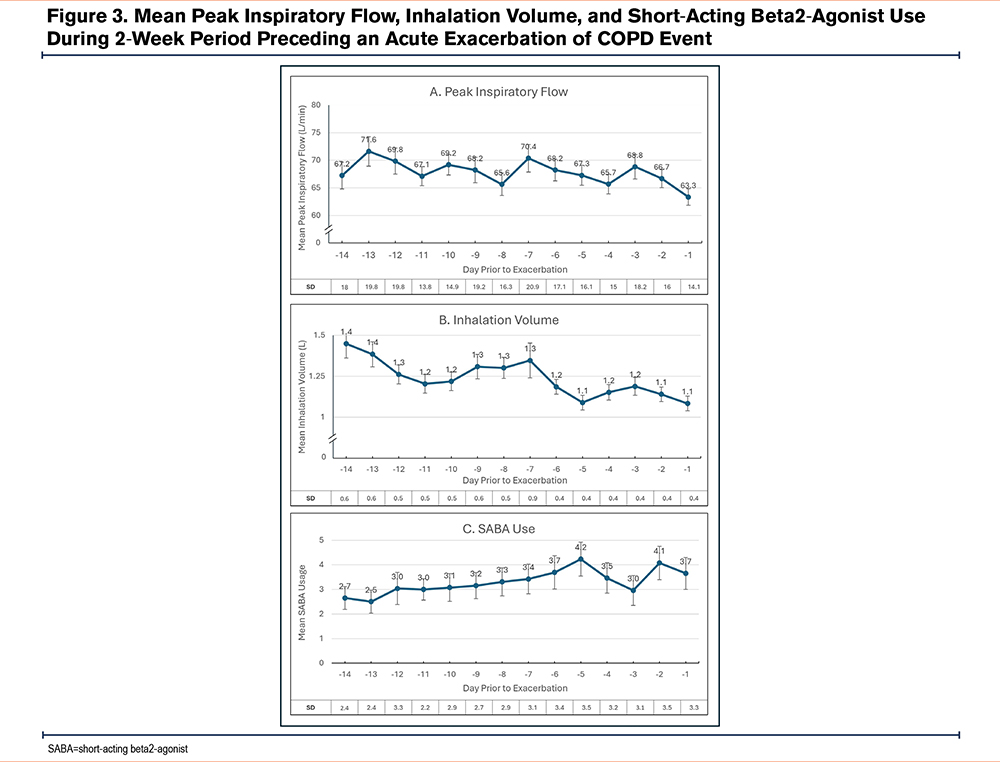
A total of 15 participants experienced 23 AECOPD events during the study. Several inhaler metrics captured by the digital inhaler worsened in the 14-day period prior to the onset of an AECOPD event (Table 2). There were statistically significant reductions in mean inhalation volume, mean inhalation duration, and mean time to peak during the period encompassing 14 days before the recorded AECOPD event (p<0.02), along with an increase in mean number of SABA inhalations per day (p<0.2). Specifically, mean PIF decreased 5.8%, mean inhalation volume decreased 25.2%, and the mean number of SABA inhalations increased 37.7% (Figure 3). The magnitude of change in PIF during the 14 days leading up to a reported AECOPD was minimal (67.2 L/min and 63.3 L/min, respectively [p=0.1]). While the PIF values were relatively stable across the 14 days prior to an AECOPD (Figure 3A), there was an initial decrease in inhalation volume from day -14 to -10, followed by a plateau until day -7, at which a sharper drop in inhalation volume occurred which was sustained up to the onset of an AECOPD (Figure 3B). This contrasts with rescue albuterol use, which demonstrated a gradual, steady increase from day -14 to -5, followed by a slight decrease and then a sharper uptick in the 48 hours preceding the AECOPD onset (Figure 3C).
Discussion
In this pilot study of the ProAir Digihaler in a COPD population, enriched for AECOPDs, we made several key findings. First, there was a strong correlation between self-reported and actual albuterol use during the study period. Second, rescue albuterol use, but no other inhaler metrics, correlated with self-reported symptom burden on a day-to-day basis. Third and most notably, the results demonstrate that there was variation in digital inhaler metrics, specifically reduction in inhaled volume, inhalation duration, and to a lesser extent, PIF, in the 14 days preceding an AECOPD event. These findings demonstrate the value of using digital inhaler data to monitor SABA use and the potential to use digital inhaler data to help identify AECOPD events prior to onset. Lastly, this study demonstrates the ability to conduct decentralized trials in patients with ambulatory COPD using digital inhaler technology.
Remote patient monitoring systems have altered the landscape of disease assessment and control in chronic illnesses. The existing literature regarding remote patient monitoring in COPD includes interventions with nonspecific assessments (blood pressure, pulse oximetry) or respiratory assessments via spirometry or oscillometry,20 with data managed asynchronously or synchronously.21 Other devices include ear pieces and adhesive sensors that record not only heart and respiratory rate, but also breathing pattern, oxygen saturation, cough, temperature, activity, and sleep.14 Electronic monitoring inhalation devices were initially developed to inform patient adherence, but advances in technology, including integration of pressure sensors and Bluetooth technology, have permitted more robust assessments of respiratory physiology.22 More advanced devices provide data from symptoms, device use, and location, integrated with weather, pollen counts, and pollution, and have been shown to improve asthma outcomes.23 In previous research in asthma, the ProAir Digihaler has been shown to improve disease control, reduce SABA use, and increase the number of SABA-free days.17 This is the first report to describe the results of real-world remote deployment of a digital inhaler device and a companion app with symptom collection in patients with COPD.
As of June 2024, the software and technical support for Digihaler App were discontinued in the United States. Although the ProAir Digihaler is no longer commercially supported, there are other digital inhaler technologies that can be used to monitor SABA use. This current pilot study highlights the utility and importance of objective digital inhaler SABA data in COPD, especially preceding an AECOPD. There are few reported data regarding the changes in rescue medication use and inhalation metrics preceding an AECOPD.15,16 Characterizing these changes informs not only the physiological changes preceding an AECOPD but can also serve to automatically alert a patient or provider about an impending AECOPD. Early detection and treatment of AECOPDs have been shown to improve outcomes in AECOPDs. In this study, we observed qualitative and quantitative changes in inhaler metrics and rescue use patterns in the 14 days prior to an AECOPD onset. Interestingly, the most substantial physiological changes were in inhalation volume, inhalation duration, and time to peak inhalation, while changes in PIF were nominal. Inhalation volume changes were observed as early as 10 days prior to an AECOPD, and were temporally associated with increases in ProAir use, suggesting that physiological changes were perceptible to the participants (as represented by increased rescue inhaler use). It is notable that the largest increase in rescue inhaler use occurred approximately 2 days before the AECOPD onset, while a substantial decline in inhalation volume was observed 7 days prior to the AECOPD onset. Time to peak inhalation has been shown to impact drug delivery. The observation of changes in time to peak prior to an AECOPD onset may indicate that changes in drug delivery are occurring in the critical window before AECOPD onset.24 A previous study showed that the inhaler metrics measured by the ProAir Digihaler can be used to create a predictive model for AECOPDs.25 The results from this study suggest that an algorithm incorporating physiological changes may identify impending AECOPDs sooner than algorithms relying solely on increased rescue inhaler use. Future analyses of the data collected in this study will include identifying algorithmic alerts incorporating both physiological and inhaler use changes to best predict AECOPD events.
The data presented here show that the ProAir Digihaler provides an objective measure of rescue inhaler use and increases in use herald an impending AECOPD. This may be beneficial, particularly for poor perceivers of symptoms or patients who do not keep track of their rescue inhaler use. Interestingly, in our study, there was a strong correlation between self-reported albuterol use assessed monthly via phone visit and actual use captured by the Digihaler. Not all patients track their inhaler use, and patients who use inhaler technologies with accompanying apps may become more aware of their inhaler use simply due to having access to this data on their smartphones. The inhaler use data informs the relationship between patient behavior and patient reporting, providing useful data for providers to consider when assessing a patient. These results differ from prior studies showing that self-report is a poor surrogate for actual use. Our findings may differ from previous studies because participants in our study were aware that the Digihaler tracks actual use, which could have led to more robust reporting by those enrolled in this study. In our study, participants completed a daily self-assessment using a Leikert scale response to a question about how they were feeling. When comparing correlations between Leikert responses and Digihaler data, rescue albuterol use, but not inhaler metrics, correlated with self-reported symptom burden on a day-to-day basis. Integrating these data with the pre-AECOPD observations, one can infer that frequency of rescue inhaler use can serve as a useful surrogate for patient perception of disease variability in the stable state, but inhalation metrics, such as inhaled volume, may be more informative in the pre-AECOPD period.
This study has limitations. This was an exploratory pilot study designed without prespecified power calculations for reasons previously described. The study was conducted during the immediate postpandemic era when social distancing measures and remote monitoring of patients were still in place, and fewer patients were participating in clinical trials overall. The requirements for smartphone ownership and operational skills further restrict the device's widespread use to a small group of individuals. The study had a higher-than-anticipated dropout rate, and lacked a control group, potentially impacting the findings of this pilot study. However, the final analytical dataset still provided more than 9000 inhalation events and >20 AECOPD events for analysis. AECOPD events were captured via self-report at monthly phone visits. The use of monthly visits reduced the chance for recall bias, but relying on self-report of AECOPD events may have decreased the fidelity of onset dates. The recruited population was all active smokers, and the impact of smoking on inhalation metrics remains unclear. While gender was balanced, non-White groups were underrepresented in the study, therefore, the findings may not be generalizable to other demographic groups.
In conclusion, we have demonstrated that there is a decrease in inhalation volume, inhalation duration, and time to peak, and an increase in rescue inhaler use preceding AECOPD events by as much as 14 days, which can potentially be used in the clinical setting to identify impending AECOPDs. Albuterol objectively measured by a digital inhaler strongly correlates with patients’ self-reported SABA use. These findings demonstrate the potential to use digital inhaler to improve the assessment and care of COPD patients in the chronic setting and during acute decompensation.
Acknowledgements
Author contributions: MBD, TH, AB, DY, and JAO made substantial the contributions to the conception and design of the work, analysis and interpretation of data, and drafting of the work, and approve of the final version and agree to be accountable for all aspects of the work. CCH made substantial contributions to the acquisition of data, and reviewing the draft critically for important intellectual content, and approves of the final version and agrees to be accountable for all aspects of the work.
Data Sharing: The data sets used and/or analyzed for the study described in this manuscript are available upon reasonable request. Qualified researchers may request access to patient level data and related study documents including the study protocol and the statistical analysis plan. Patient level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please visit www.clinicalstudydatarequest.com to make your request.
Declaration of Interests
JAO has received research grants from Teva, Boeringer Ingelheim, and Sepracor. She has served as a consultant for Cheisis, numerous law firms, Astra Zeneca, Verona, Viatris, Theravance, and Boeringer Ingelheim.
MBD reports grants paid to his institution from the National Institutes of Health, the Patient-Centered Outcomes Research Institute, Vertex, the American Lung Association, Boehringer-Ingelheim, and Midmark unrelated to this work. He reports personal consulting fees from AstraZeneca, Verona, Takeda, Becker Pharma, Boehringer-Ingelheim, Strados, and GlaxoSmithKline unrelated to this work. TH, AB, and DY are employees of Teva Pharmaceuticals. CCH reports no conflicts of interest.