Running Head: Nail Cortisol as a Marker of Stress in COPD
Funding Support: T.M.Parekh was supported by a K23 grant from the National Heart, Lung, and Blood Institute.
Date of Acceptance: May 7, 2025 | Publication Online Date: May 8, 2025
Abbreviations: AECOPD=acute exacerbation of COPD; aOR=adjusted odds ratio; CAT=COPD Assessment Test; CI=confidence interval; COPD=chronic obstructive pulmonary disease; CRP=C-reactive protein; ED=emergency department; ELISA=enzyme-linked immunosorbent assay; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; HPA=hypothalamic-pituitary-adrenal; ICS=inhaled corticosteroids; IL-1β=interleukin 1 beta; IL-6=interleukin 6; IQR=interquartile range; mMRC=modified Medical Research Council; OR=odds ratio; PHQ-2=Patient Health Questionnaire 2; PRAPARE=Protocol for Responding to and Assessing Patients' Assets, Risks and Experiences; SD=standard deviation; SDH=social determinants of health; UAB=University of Alabama
Citation: Parekh TM, Ramachandran R, Kim Y, et al. Psychobiologic correlates of stress in individuals with COPD. Chronic Obstr Pulm Dis. 2025; 12(3): 213-222. doi: http://doi.org/10.15326/jcopdf.2024.0578
Online Supplemental Material: Read Online Supplemental Material (590KB)
Introduction
There is mounting evidence that individuals with diseases of chronic inflammation, including chronic obstructive pulmonary disease (COPD), coronary artery disease, asthma, and autoimmune diseases, are more susceptible to the deleterious effects of chronic stress.1,2 Under states of chronic stress, cortisol, the primary neuroendocrine stress hormone, loses its ability to regulate inflammation. Inflammatory cytokines, including tumor necrosis factor-1, interleukin-6 (IL-6), and interleukin-1 beta (IL-1β), are activated by the disruption of the hypothalamic-pituitary-adrenal (HPA) axis and by stimulation of the sympathetic nervous system. A prolonged increase in cortisol increases glucocorticoid resistance, leading to unregulated inflammation, suppressed cellular immunity, and heightened vulnerability to respiratory viruses.3,4 Our prior work has found an association between increased levels of stress and risk of severe COPD exacerbation.5 While the benefit of stress reduction on exacerbation risk remains unknown, stress reduction may be a key nonpharmacologic therapy for improving quality of life in patients with chronic diseases, including COPD. In addition, the concept of grit, defined as “passion and perseverance towards long-term goals,” has been studied in the positive psychology field and may have a significant impact in mitigating the negative effects of stress on our health.6 Grit has been associated with lower levels of stress and burnout in other populations, yet has not been studied in COPD.7,8
Psychological intervention studies frequently use serum or salivary cortisol and other inflammatory markers as surrogate biologic outcomes for the effectiveness of their stress reduction interventions.9,10 However, the use of serum or salivary cortisol as a marker of stress in COPD encounters some specific limitations. First, tobacco use can acutely increase serum cortisol, which may overestimate levels of stress.11 Second, treatment of acute exacerbations of COPD (AECOPDs) with steroids can suppress cortisol production, which may take up to 14 days after treatment to recover.12,13 Prior research in cancer patients has found that measurement of cortisol levels from nail clippings, a novel measure of physiologic stress, provides an assessment of chronic cortisol accumulation, approximately 4–5 months prior.14 A case-control study of 162 men in Japan who experienced acute coronary syndrome has also tied levels of nail cortisol with a 2-fold increase in the risk of acute coronary syndrome.15 The utility of nail cortisol levels in the measurement of chronic stress and the association with clinical outcomes in individuals with COPD is unknown, but may avoid the limitations that are present with salivary and serum testing, including their diurnal pattern of release and intra- and inter-day variations. While cortisol may be measured from hair samples, many patients dislike hair samples for cultural reasons or for lack of hair, which is common in the elderly COPD population, presenting an additional advantage to the use of nail clippings.
To determine the utility of nail cortisol as a biologic measure of chronic stress in COPD, we evaluated the relationship between nail cortisol and perceived stress in individuals living with COPD. We also examined associations of psychobiologic measures of stress (perceived stress, serum cortisol, nail cortisol) with demographic, clinical, and psychological variables, including grit, in COPD to determine if there were similar associations across measures of stress. We hypothesized that nail cortisol would be a reliable biologic measure of perceived stress in COPD and that associations of nail cortisol with demographic, clinical, and psychological variables would be similar to other measures of stress (perceived stress and serum cortisol).
Methods
Study Design
This study is a cross-sectional study of individuals with COPD conducted at the University of Alabama at Birmingham (UAB). The UAB Institutional Review Board approved the study. Written consent was obtained from all participants.
Participant Selection and Setting
Participants were recruited from the UAB Lung Health Center and UAB pulmonary clinics from November 2019–May 2022. Individuals were included in the study if they had a smoking history of ≥10 pack years (either current or former smoker) with spirometry meeting COPD criteria (forced expiratory volume in 1 second [FEV1] to forced vital capacity ratio <0.7),16 were able to read and write independently, and were able to speak English. Exclusion criteria included major cognitive impairment, diagnosis of interstitial lung disease, daily use of oral corticosteroids, or if they were treated for a COPD exacerbation in the 4 weeks prior to the research visit.
Measurements
This study involved the collection of primary data using surveys, blood samples, and nail samples, which were collected at a single time point. Our primary psychobiological measures of stress included nail cortisol, serum cortisol, and perceived stress.
Nail Samples
Participants were instructed to have 2 weeks of fingernail growth prior to the baseline visit. They had 10 fingernails clipped during the baseline assessment and stored in labeled plastic bags at room temperature. Nail cortisol levels were processed at the Bio-Analytical Redox Biology Core lab at UAB using a slightly modified procedure as described by Warnock and Meyer et al.17,18 Nail clippings were washed twice with 2ml isopropanol and dried overnight. Then, 50mg of nails were ground into a powder by 5mm steel grinding balls, combined with methanol (1ml per 50mg of powdered nail), and placed on a rotator for 18 hours to extract the cortisol. Samples were centrifuged, and cortisol levels were measured using enzyme-linked immunosorbent assay (ELISA) kits. If samples remained outside of the range for the standard curve after being rerun they were excluded from analysis (n=4). Samples were run in 2 separate batches (n=33, n=23). The inter-assay coefficient of variation was 6.9% for the high standard and 0.8% for the low standard for the first batch, and 5.3% for the high standard and 3.7% for the low standard for the second batch.
Blood Samples
Cortisol levels were determined by enzyme immunoassay. Serum cortisol was measured from a blood draw before 10 AM, and participants were asked to refrain from exercise, caffeine use, tobacco use, alcohol use, and sleep loss prior to a fasting blood draw. Fibrinogen, C-reactive protein (CRP), IL-1β, and IL-6 were also measured using commercially available ELISA kits.
Surveys
Perceived stress over the prior month was measured using Cohen’s Perceived Stress Scale, a 10-item instrument with a score ranging from 0–40 and higher scores indicating higher levels of stress; psychometric properties indicate adequate reliability (Cronbach's Alpha coefficient 0.78)19,20,21 Life events were measured from the Life Events Checklist, which assessed total negative and positive life events that occurred over the prior 12 months.22,23 For our analysis, we only included total negative life events as we expected the experience of negative events to influence chronic stress. Grit was measured by the Short Grit Scale, an 8-item instrument with scores ranging from 1–5, with higher scores indicating higher levels of grit or resilience.6 Depression was measured using the Patient Health Questionnaire–2 (PHQ-2), which includes 2 questions about the frequency of anhedonia and depressed mood over the prior 2 weeks. A score of 3 or greater (scale 0–6) indicates a major depressive disorder is likely.24 Social determinants of health (SDH) were measured by the Protocol for Responding to and Assessing Patients' Assets, Risks and Experiences (PRAPARE) questionnaire, a 21-question survey assessing SDH domains including housing, transportation, and financial insecurity.25 Based on answers to this questionnaire, we derived whether an individual experienced financial insecurity. This was defined as being unable to afford either food, utilities, clothing, phone, childcare, medicine, or health care. We also determined level of social support from the PRAPARE questionnaire which asks “How often do you see or talk to people that you care about and feel close to (less than once a week, 1–2 times per week, 3–5 times per week, 5 or more times per week)?” Health status was assessed by the question “In general, how would you rate your health (excellent, very good, good, fair, poor)?” The impact of COPD symptoms over the prior week on quality of life was measured by the COPD Assessment Test (CAT) (8-item instrument; score range 0–40 with higher scores indicating more symptoms).26 Dyspnea was measured with the modified Medical Research Council instrument, which measures degree of breathlessness.27 In addition, participants took a comprehensive survey that inquired about medication use, COPD-related symptoms, health care utilization for AECOPDs over the prior 12 months, comorbidities, and tobacco use.
Statistical Analysis
Descriptive statistics for sociodemographic and clinical characteristics, including stress measures of study participants, were tabulated for the total population. Pearson’s correlation was used to estimate the relationship between nail cortisol and perceived stress, serum cortisol, and inflammatory biomarker measurements. We used a multivariable linear regression model to evaluate the association between perceived stress and nail cortisol, controlling for confounders that may affect cortisol levels over time including current tobacco use, use of anxiety or depression medications, and use of antihypertensive medications. Linear and logistic regression models were used to assess the relationship between individual demographic (age, sex, race, financial insecurity), clinical (symptoms as measured by the CAT, moderate or severe exacerbations, health status, smoking status, fibrinogen, IL-1β, IL-6, CRP), and psychological (depression [PHQ-2], grit [Short Grit Scale], negative life events [Life Events Checklist]) covariates determined a priori to be important in psychological stress and the following outcomes: levels of perceived stress, serum cortisol, and nail cortisol. For the logistic regression model, median values of perceived stress, serum cortisol, and nail cortisol were used to determine “high” versus “low” levels of stress. A stepwise logistic regression approach was applied to select the optimal set of predictors while avoiding potential multicollinearity issues among the tested variables, using entry and stay P-values of less than 0.05. Demographic variables were chosen a priori to remain in the model. All statistical analyses were performed using SAS 9.4. Results were determined to be statistically significant when the accompanying statistical test yielded a 2-tailed probability of less than 0.05.
Results
A total of 50 participants had valid nail cortisol data available and were included. Table 1 displays baseline characteristics of the cohort. The cohort included 42% women and 40% non-White individuals with a mean (standard deviation [SD]) age of 65±8. Most individuals were former smokers (52%), and 14% experienced financial insecurity. The cohort had a mean (SD) FEV1 of 50+/-17% predicted, and a majority had either moderate or severe airflow obstruction (98%). Approximately 1/5 of the cohort (19%) was on long-term oxygen therapy. In the prior 12 months, 46% of individuals had a moderate exacerbation and 28% had a severe exacerbation (defined as an emergency department [ED] visit or hospitalization). The cohort had a high symptom burden (CAT mean 20) and a high prevalence of acid reflux (50%), anxiety (38%), and depression (36%). The median (interquartile range [IQR]) nail cortisol level was 0.016 (0.03)nmol/g, median (IQR) serum cortisol was 9.1 (5.3)mcg/dl, and the median (IQR) perceived stress level was 12 (9), which is in the lower tertile of the scale’s score, however, similar to normative scores for the general population over 45 years old (45 and over: PSS 11.9–12.6).21 Supplemental Tables 1a-1c in the online supplement describe baseline characteristics of the cohort based on median (low and high) levels of perceived stress, serum cortisol, and nail cortisol, respectively. Individuals with high perceived stress were more likely to be women, have a higher symptom burden, fair or poor health status, higher PHQ-2, more negative life events, and lower levels of grit compared to individuals with lower perceived stress. Individuals with high serum cortisol were more likely to be male, have coronary artery disease and sleep apnea, and have higher fibrinogen and grit levels. Those with high nail cortisol had lower rates of exacerbations (total, moderate, and ED visits) and a higher likelihood of having acid reflux and osteoarthritis.
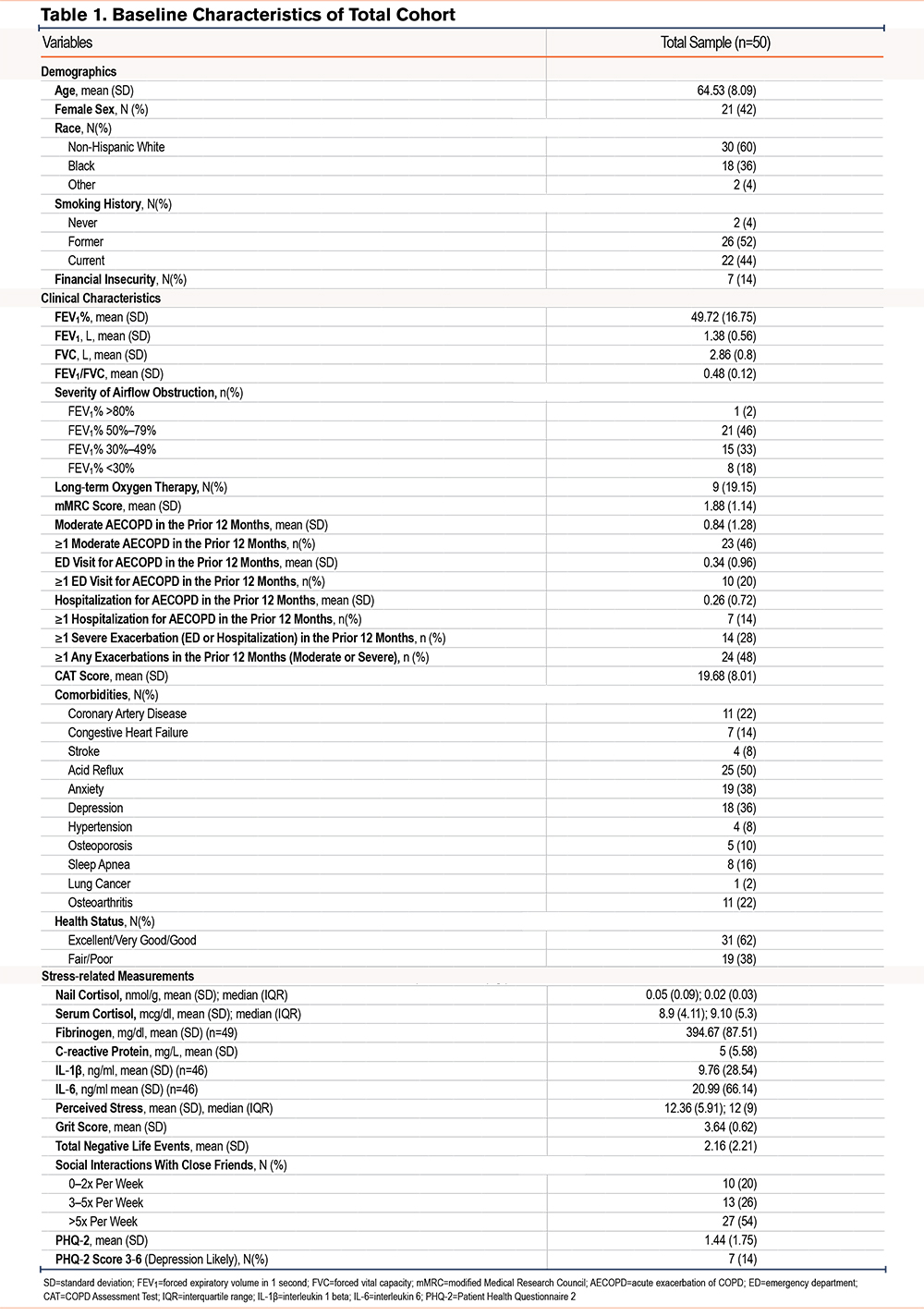
We found that nail cortisol was not significantly correlated with perceived stress (Pearson’s r=-0.11; p=0.45), serum cortisol (Pearson’s r=0.04; p=0.76), or any other inflammatory biomarkers (Table 2, Supplemental Figure 1 in the online supplement). After controlling for current tobacco use, use of anxiety or depression medications, and use of antihypertensive medications, we did not find a significant association between perceived stress and nail cortisol (Beta (standard error)=-0.001 (0.002); p=0.8).
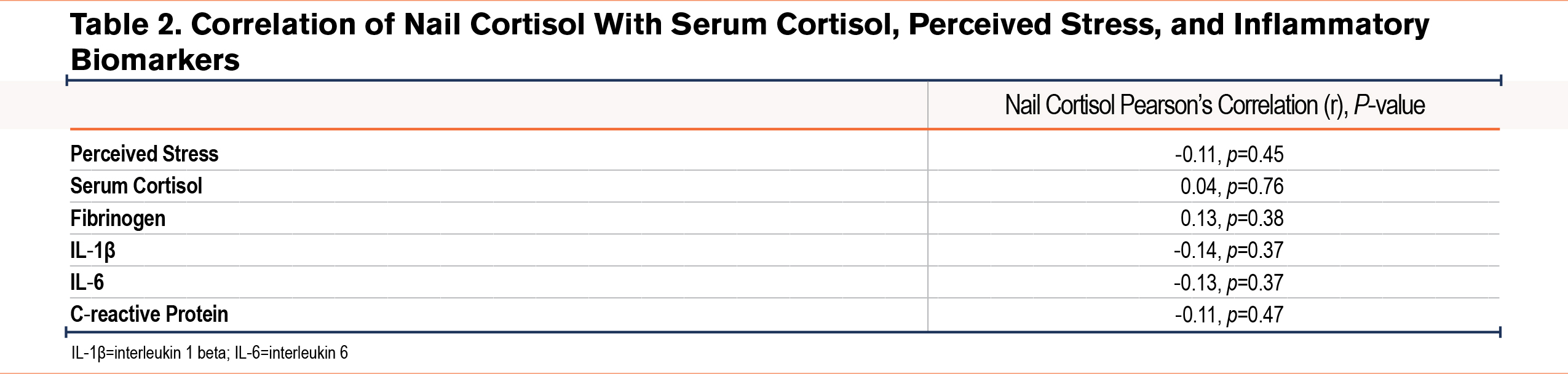
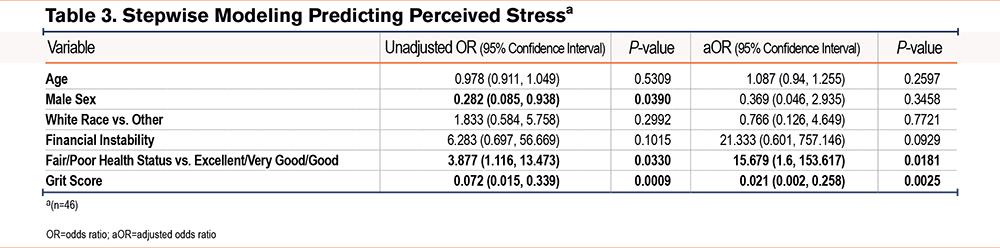
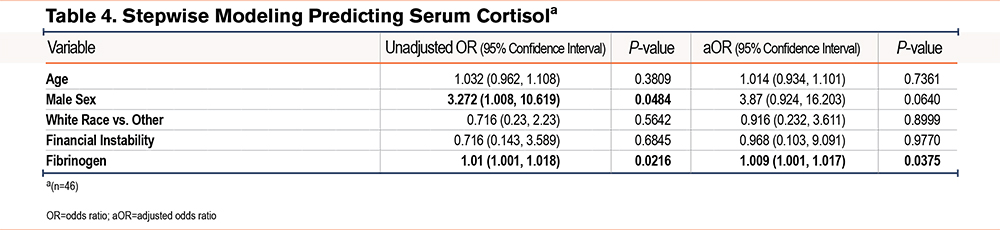
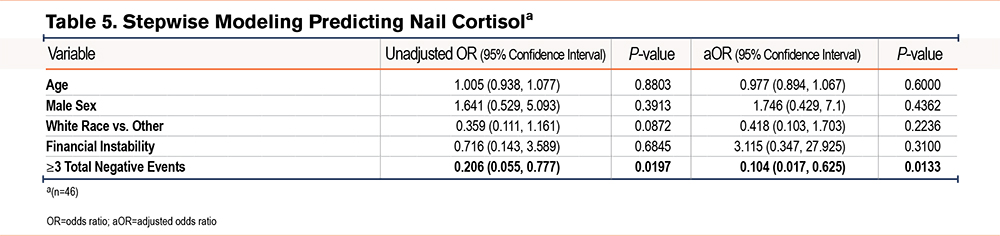
Supplemental Table 2 in the online supplement shows the results of univariate linear regression models of perceived stress, serum cortisol, and nail cortisol. Results indicated that a higher CAT score, a higher PHQ2, financial insecurity, and more total negative life events were associated with higher perceived stress levels while higher grit and male sex were associated with lower perceived stress levels. There were no associations in the univariate analyses of covariates with serum cortisol; the direction and strength of nonsignificant associations were not similar compared to associations with perceived stress or nail cortisol. Nail cortisol had a weak negative association with total negative life events. Table 3, Table 4, and Table 5 show the unadjusted and adjusted odds ratios of covariates predicting perceived stress, serum cortisol, and nail cortisol, respectively. Health status (fair/poor versus excellent/very good/good) was associated with increased odds of having high perceived stress (OR [95% confidence interval (CI)], 15.7 [1.6, 153.6]) while each point higher on the grit score (indicating more grit) was associated with 98% lower odds of having high perceived stress (OR [95% CI], 0.02 [0.002, 0.258]). Fibrinogen was significantly associated with serum cortisol [OR (95% CI), 1.009 (1.001, 1.017)]. Total number of negative events (3 or more versus 0–2) was significantly associated with lower odds of having high nail cortisol (OR=0.104, 95%CI: 0.017, 0.625). There were no similarities in associations between demographic, clinical, and psychological variables and psychobiological correlates of stress.
Discussion
In this pilot study, we found that nail cortisol was not correlated with perceived stress, serum cortisol, or inflammatory biomarkers, and there was no association between nail cortisol and perceived stress in a model controlling for confounders. Moreover, there were no similarities in associations between demographic, clinical, and psychological variables and various measures of stress. The total number of negative life events was the only significant covariate associated with nail cortisol, however, unexpectedly, there was a negative association such that individuals with a greater number of negative life events had a lower level of nail cortisol. Our study suggests that the relationship between perceived stress and its biological consequences in COPD is complex and likely influenced by several unmeasured confounders.
Psychological stress in COPD is underrecognized and undertreated. In prior work, we have found that individuals sampled from a pulmonary clinic who were living with COPD and had high stress levels were more likely to rate their health as “poor” and had a higher burden of symptoms based on the CAT scores. This “high stress” group was also twice as likely to have severe exacerbations in the prior 2 years. When accounting for low income, those with high stress had 4-fold increased odds of severe exacerbations of COPD.5 The ability to identify and treat patients at risk for unregulated or chronic stress is an integral factor that can lead to improved quality of life and possibly improved clinical outcomes.
Understanding how to measure chronic stress in COPD is the first step in being able to identify patients at risk for its negative downstream effects. Since the seminal papers by Warnock et al and Meyer et al were published in 2010,17,18 the current literature on nail cortisol as an emerging chronic stress biomarker has evolved with several exploratory studies showing mixed results with regards to correlations with other known measures of stress. While the causal pathway between perceived stress, hypercortisolism, and a subsequent increase in nail cortisol is logical, our results are consistent with other exploratory studies published, which did not find a correlation with perceived stress and nail cortisol.28,29,30 In a study of 123 middle-aged workers, perceived stress was not associated with nail cortisol levels taken at a single time point. While the cross-sectional study design of this study, similar to ours, may have limited the evaluation of nail cortisol with past perceived stress,28 a longitudinal study of 47 low-income mostly Black (97%) adolescents also found no correlation between perceived stress assessed at baseline and nail cortisol assessed at a 2 month follow up.30 The absence of a significant relationship between perceived stress and nail cortisol is similar to findings in studies examining perceived stress and hair cortisol, another biological measure of chronic stress.31 This may be due to the inherent limitations of survey-based assessments, which can be affected by social desirability, prestige, or central tendency bias. Cross-cultural differences in the interpretation of stress may also introduce bias when using survey-based assessments.
While positive associations between experience of stressful life events and elevated nail cortisol levels have been shown in other populations (middle age workers, Serbian refugees),28,32 in our study, we found that more negative life events were associated with lower nail cortisol levels. While surprising, negative associations between stressful life events and nail cortisol have been seen in high-stress populations. A study of Australian Indigenous (n=179) and non-Indigenous (n=66) young adults found that the Indigenous group had a higher number of stressful life events compared to the non-Indigenous group (median 6.0; IQR 4, 9 versus 1.0; IQR 0, 2; p<.001). In a multivariable regression analysis examining associations with nail cortisol, there was a strong association of Indigenous status and nail cortisol level (geometric mean 1.82; 95% CI: 1.07–3.09, p=.027). However, when examining the relationship between stressful events and nail cortisol level by Indigenous status, the study found that Indigenous young adults had a 11% lowering of fingernail cortisol levels for each stressful event (GM=0.89; 95% CI: 0.84–0.95; p=.001) while there was no significant association for non-Indigenous young adults. The authors of this study postulated that Indigenous young adults are under states of severe chronic stress, which may exhaust their stress response, leading to a hypoactive state of cortisol production.33 This diminished functionality of the HPA axis leading to hypocortisolism is documented in healthy individuals who experienced traumatic childhood events as well.34,35 Similarly, individuals with chronic stress and COPD may live under states of heightened systemic inflammation, both from their underlying lung disease and from heightened stress. Over time, their ability to produce cortisol may be diminished, which may explain the correlation between increased number of negative life events and lower nail cortisol.
Our study results should be interpreted considering several limitations. First, measurements were taken at a single time point, therefore, nail cortisol could not be compared to preceding measurements of perceived stress or serum cortisol to determine causality. Cortisol can be deposited into nail beds either by deposition into the nail matrix or by diffusion into the nail bed. Deposition into the nail matrix can reflect cortisol released up to 6 months prior while diffusion into the nail bed can reflect cortisol from 1 week prior.36 The temporal reflection of cortisol release into the nail bed still requires further understanding and has implications for the methodology in future nail studies. Second, our sample size was small, taken from a single academic medical center, and the median perceived stress level was in the lower tertile of the scale, which was near the normative values of perceived stress. Results may not be generalizable in a sample of highly stressed individuals who have experienced chronic dysregulation of their HPA axis affecting cortisol production. Third, data on exacerbations was self-reported, which is limited by poor recall. Fourth, the Life Events Checklist asks about life events that occurred anytime in the prior 12 months; however, we did not have information on the timing of life events to determine if they were within the period that would reflect nail cortisol accumulation (4–5 months). Fifth, we did not have data on the presence of endocrine disorders, which may have impacted cortisol levels. Finally, while our study excluded individuals who had an exacerbation in the prior month or were taking daily oral corticosteroids, it is unclear how treatment of exacerbations with oral corticosteroids in the preceding months (>1 month) may have affected nail cortisol levels. In addition, as inhaled corticosteroids (ICS) are widely used as part of triple therapy for COPD patients, this was not an exclusion criterion for our study and may have influenced our results as ICS can suppress serum cortisol levels, which may underestimate stress levels in an individual.37 Regardless of these limitations, this is the first study to test the utility of nail cortisol in a sample of individuals with COPD, and we provide a foundation upon which future studies can further investigate nail cortisol as a chronic stress biomarker.
In conclusion, in this cross-sectional study, nail cortisol was not a reliable marker of chronic stress in individuals living with COPD. Future research should examine the prospective relationship of perceived stress and nail cortisol, examine the utility of nail cortisol in a high-stress population, and determine if nail cortisol is predictive of important clinical outcomes in COPD including exacerbations and cardiovascular events.
Acknowledgements
Author contributions: TMP, MTD, JMW, and YK contributed to the conception and/or design of the study. TMP, MTD, and JMW contributed to the acquisition of the data. TMP, MTD, RR, YK, EM, DB, and ZH contributed to the data analysis and/or interpretation. TMP, MTD, JMW, RR, YK, DB, EM, and ZH contributed to writing the article and had substantial involvement in its revision prior to submission.
All authors have significantly contributed to the intellectual content of the article and gave final approval of this manuscript to be published.
Other acknowledgments: We would like to acknowledge Melissa J. Sammy, PhD, and Kelley E. Smith-Johnston, BS, at the UAB Bio-Analytical Redox Biology (BARB) Core, DRC (NIDDK P30DK079626), Heersink School of Medicine, NORC (NIDDK P30DK056336), and CCTS (NIH UL1TR003096), for their assistance with measuring nail cortisol levels.
Declaration of Interests
TMP is supported by the National Heart, Lung, and Blood Institute (K23HL153672). JMW served on an advisory board for GSK, Takeda, AstraZeneca, Bavarian Nordic, Krystal Biotech, Sanofi, and Verona Pharma; holds patent PCT/GB2021/050658 with Mereo BioPharma; received research support from the Alpha-1 Foundation, ARCUS-Med, the Department of Veterans Affairs, GlaxoSmithKline, Grifols, InhibrX, Medscape, Mereo BioPharma, NIH/NHLBI, Takeda, InhibrX, the American Lung Association, and Verona Pharma; and holds stock in Alveolus Bio. MTD has received consulting fees from Aer Therapeutics, Aprea, AstraZeneca, Genentech, GSK, Novartis, Pulmonx, and Teva; received royalties from UptoDate; received travel support from GSK; received research support from the Department of Defense, the American Lung Association, and the National Institutes of Health. ZH, EM, YK, RR, and DB have no conflicts of interest to declare.