Running Head: Inhalation Innovation: Optimizing COPD Care
Funding Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Date of Acceptance: March 21, 2025 | Publication Online Date: April 1, 2024
Abbreviations: BMQ=Beliefs about Medicines Questionnaire; COPD=chronic obstructive pulmonary disease; DPI=dry-powder inhaler; EHR=electronic health record; GOLD=Global initiative for chronic Obstructive Lung Disease; HCPs=health care professionals; ICS=inhaled corticosteroids; IQR=interquartile range; LABA=long-acting beta2-agonists; LAMA=long-acting antimuscarinic; mMRC=modified Medical Research Council; PHARMACOP=pharmaceutical care for patients with COPD; pMDI=pressurized metered-dose inhaler; QEI=Queen Elisabeth Institute; R=quartile 1 and quartile 3; SMI=soft mist inhaler
Citation: Walravens A, Walravens E, Wuyts S, et al. Inhalation innovation: optimizing COPD care through clinical pharmacist integration in a rehabilitation hospital's multidisciplinary team – a quality improvement study. Chronic Obstr Pulm Dis. 2025; 12(3): 240-249. doi: http://doi.org/10.15326/jcopdf.2024.0569
Online Supplemental Material: Read Online Supplemental Material (591KB)
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease, a major health problem worldwide that is frequently diagnosed among long-term smokers.1,2 It is a disease in which the patient has airflow limitation due to bronchitis and emphysema.3,4 Patients with COPD often experience exacerbations, characterized by a worsening of the illness, resulting in a lower quality of life.5
Chronic inhalation therapy is prescribed depending on the stage of the disease and further fine-tuned based on individual needs and preferences. Several classes of pharmacological agents can be administered to offer symptom relief and minimize exacerbations.6 Various devices exist to administer these drugs, such as pressurized metered-dose inhalers (pMDI), breath-actuated metered-dose inhalers, dry powder inhalers (DPI), soft mist inhalers, and nebulizers. As there are a variety of devices, and consequently a variety of distinct inhalation techniques, sufficient comprehension of their administration is crucial to assure treatment success.7-10 Inhalation errors may result in either the absence of drug administration or its improper delivery, potentially exacerbating symptomatic manifestations of the underlying disease.8,11,12 Moreover, a considerable proportion of individuals face challenges in adhering to inhalation therapy, whether the reasons are intentional or unintentional. Research shows that only 33.6% of patients with COPD are fully adherent to their inhalation therapy.8,13,14
Different studies have investigated the importance of a structured pharmaceutical care protocol. For example, in a community pharmacy setting, the Pharmaceutical care for patients with COPD (PHARMACOP) protocol induced improved adherence and inhalation technique. Community pharmacists provided personalized instructions on correct inhaler use, offered direct feedback, and emphasized the importance of adherence. Additionally, follow-up and monitoring ensured patients remained consistent in their medication use.15
In acute care hospitals, clinical pharmacy is already well-established and extensively studied. Research often evaluates the strengths of hospital pharmacists, e.g., to decrease polypharmacy in frail, geriatric patients. However, there has been growing attention to more specific patient populations and education in pulmonary diseases as well, including COPD.16-19 These studies have shown that targeted pharmaceutical interventions can enhance medication adherence and improve inhalation techniques, which can lead to better outcomes for COPD patients.20-22
Patients with a high risk of pulmonary complications often require more thorough follow-up and are transferred to a rehabilitation service after an acute stay in the hospital. Rehabilitation settings focus on a specific population with longer lengths of stay, presenting a vital opportunity to improve adherence and inhalation techniques. This offers great opportunities for interventions by hospital pharmacists. Despite the well-established role of pharmacists in acute care settings,21 evidence on the benefit of a clinical pharmacist in rehabilitation care remains limited.
Aim
In this study, the feasibility of the implementation of a standardized pharmaceutical care protocol for inhaler therapy in patients with COPD was evaluated in a rehabilitation hospital.
Ethics Approval
Ethical approval was obtained with the Committee of Medical Ethics of the University Hospital Brussel (Universitair Ziekenhuis Brussel); EC number: 2021–385. The included patients and health care professionals (HCPs) involved in the focus group signed an informed consent form.
Methods
Study Design and Setting
This quality improvement study (mixed-methods) first focused on implementing a pharmaceutical care protocol to optimize inhaler therapy for patients with COPD, managed by a clinical pharmacist. Secondly, the feasibility of routine implementation of the pharmaceutical care protocol was evaluated in a focus group discussion with the HCPs engaged in the intervention.
Setting
The study was carried out at the Queen Elisabeth Institute (QEI - Oostduinkerke, Belgium), a 165-bed hospital with 20 beds specifically for cardiopulmonary rehabilitation. It is a rehabilitation hospital for both residential and outpatient care, located in the same physical location. Patients who are admitted are usually transferred after a stay in an acute hospital. A rehabilitation hospital is comparable to a long-term, acute care center. The hospital pharmacy team comprises a pharmacist trained in smoking cessation techniques who visits every admitted active smoker, providing personalized counseling and support as part of usual care.
Implementation of the Pharmaceutical Care Protocol
The pharmaceutical care protocol was implemented between January and May 2022. The protocol was an adapted version of the PHARMACOP protocol with more focus on a multidisciplinary approach.15 A detailed table on the content of the advanced pharmaceutical care program is provided in Appendix 1 in the online supplement.
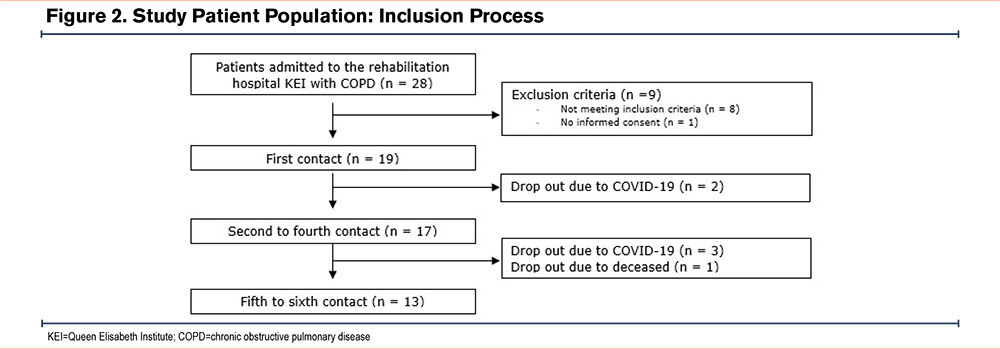
Patients could be included if they had a COPD diagnosis, were 18 years or older, used at least one inhalation medication for more than 6 months, and were Dutch-speaking. Patients with cognitive impairment or in need of isolation, e.g., due to Methicillin-resistant Staphylococcus aureus or SARS-CoV2, were excluded from the study.
After obtaining informed consent, each patient had 6 contact moments with a trained clinical pharmacist (AW or EW), as shown in Figure 1. During these contacts, inhaler therapy was assessed on multiple occasions. A detailed overview of the study protocol and educational moments can be found in Appendix 1 in the online supplement. All contacts were executed by a pharmacist. No additional inhaler education from a nurse or other health care professional took place. During the admission and counseling visits, the inhalation technique was evaluated using checklists (see Appendix 2 in the online supplement). A major error immediately caused a score of zero and was assigned when a crucial step in the inhalation technique was not correctly performed. The patients were also subjected to validated questionnaires: the modified Medical Research Council (mMRC) for dyspnea severity23 and the Beliefs about Medicines Questionnaire (BMQ), with a necessity and concerns subscale.24 An evaluation of the appropriateness of inhalation therapy was executed based on the Global initiative for Chronic Obstructive Lung Disease (GOLD)3 guidelines. The In-Check DIAL (Flexicare; Mountain Ash, Wales) was also used to assess the appropriateness of the device since it measures the highest inspiratory flow rate. It can simulate the resistance characteristics of a patient's specific inhaler and can determine if e.g., a DPI or pMDI is suited for the patient.
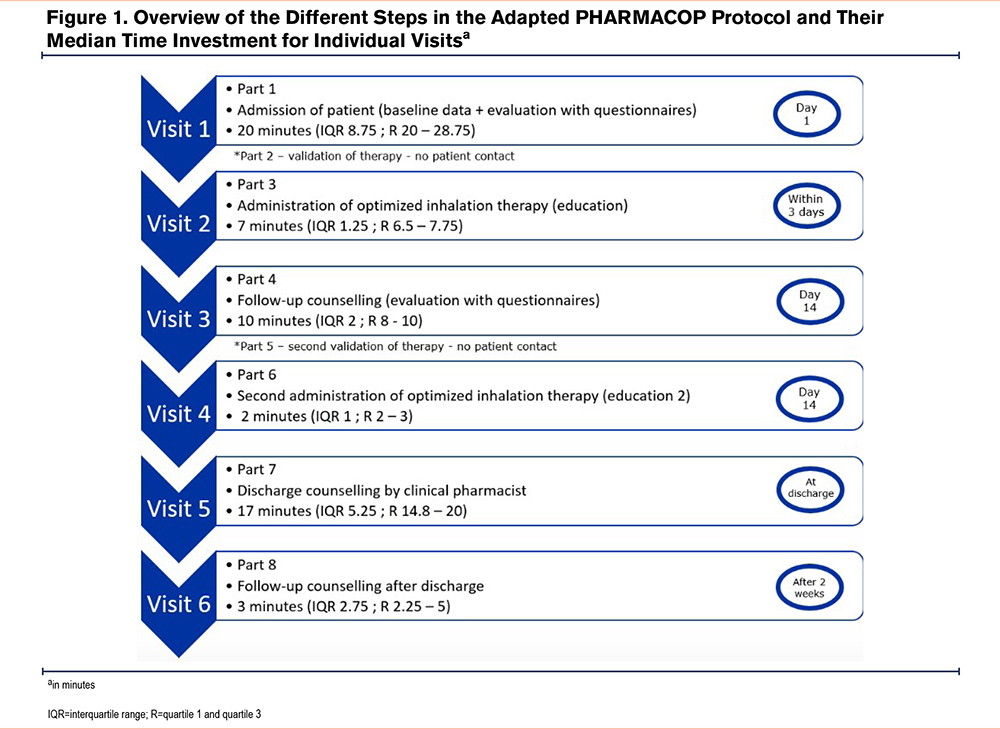
The first therapy optimization session was performed within 3 days of the baseline evaluation. Several methods were used to teach patients the correct inhaler technique. These included distributing an information brochure, showing instructional videos,25 and providing personalized guidance from the pharmacist to correct any errors in technique. The final visit at 2 weeks after discharge took place by telephone. Patients were asked to explain, step by step, how they used their inhalers.
Focus Group Discussion
Following the implementation of the care protocol, a focus group discussion was arranged on April 26, 2022, where the HCPs engaged in the project were invited to evaluate the intervention's feasibility. HCPs included 2 hospital pharmacists, 2 physicians, the responsible nurse, 2 reference nurses, the head of the nursing department, and the head of the paramedic department. The participants received the questions (Appendix 3 in the online supplement) and the protocol a few days prior to the discussion to have time to consider them beforehand.
The focus group discussion was moderated by an independent third person. The moderator used a small degree of control and guidance when there was a risk of getting off-topic. The focus group was audio-recorded.
Data Analysis
Numeric data were analyzed with Microsoft Excel 365®(Microsoft; Redmond, Washington) and IBM SPSS® (IBM; Armonk, New York). Descriptive statistics were applied. Counts were presented as frequencies and percentages. Where appropriate, averages with standard deviations or medians with interquartile ranges (IQRs) were used depending on if the distribution was normal. Data at admission were compared with those at discharge to evaluate the evolution of the patients during the hospital stay. The Wilcoxon signed-rank test was used to compare the discharge visit with the follow-up interview, assessing whether the evolution achieved during admission continued in the home setting. A per protocol analysis was used and p-values <0.05 were considered significant. The focus group discussion was written out ad verbatim in NVivo 20® (QRS International; Burlington, Massachusetts). The texts were evaluated by means of thematic analysis. We adhered to the STrenthening the Reporting of Observational studies in Epidemiology (STROBE)26 and Standards for Quality Improvement Reporting Excellence (SQUIRE)27 guidelines throughout the manuscript writing process to ensure alignment with the publication standards.
Results
Implementation of the Pharmaceutical Care Protocol
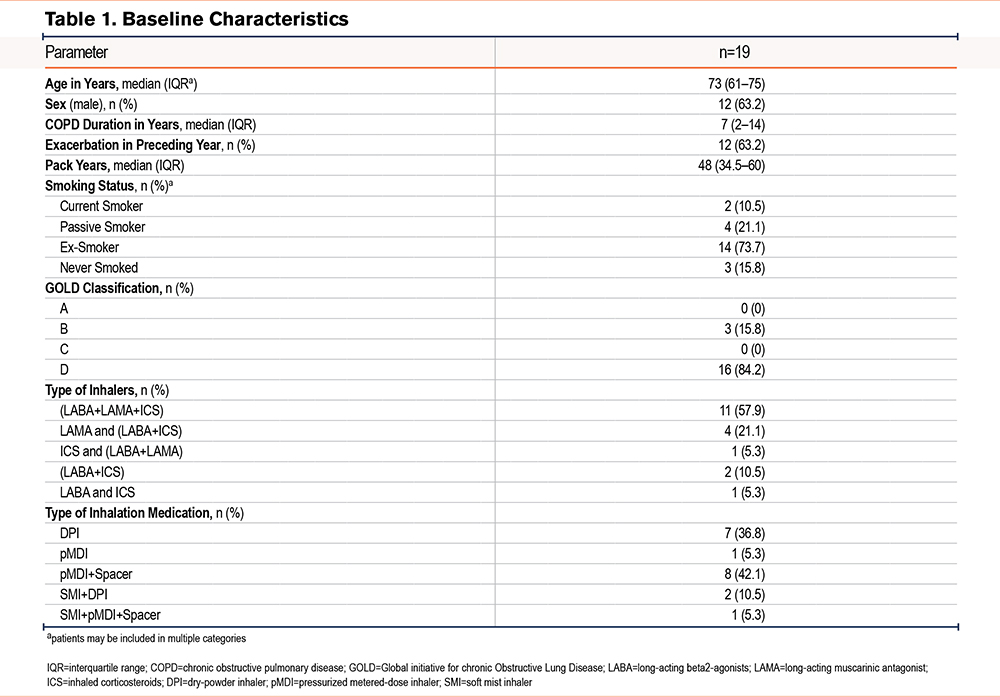
Out of the 28 patients diagnosed with COPD who were admitted to the hospital, 19 patients with COPD were included in the implementation study. Of the 9 excluded patients, only 1 refused to sign the consent form. A total of 13 patients (68%) successfully completed the entire protocol, comprising 6 contact moments. A significant drop-out rate (n=6) was caused by a SARS-CoV-2 outbreak with obligated patient isolation. The implementation process and the characteristics of the included patients are shown in Figure 2 and Table 1.
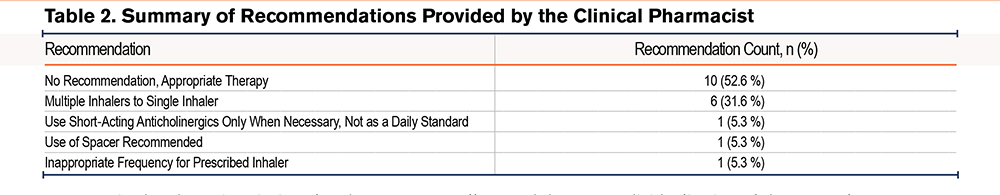
After the first assessment, a pharmaceutical recommendation was made for 9 patients (47.4%), with an acceptance rate of 66.7%. As shown in Table 2, the most common recommendation was to transition patients using 2 separate devices to combination therapy with a single inhaler. No further adjustments were made during the second therapy evaluation moment. On admission, 5 out of 13 (38.5%) evaluated patients made major errors in their inhaler use, which decreased to one patient (7.7%) at discharge and zero patients at follow-up. The median score of inhalation device use significantly improved during the patient’s hospital stay from 5.7/10 (IQR=7.1) to 9/10 (IQR=2.2) at discharge (p=0.003; z=-2.936). At follow-up, no patients exhibited a major error and their inhaler technique (median score 10/10; IQR=1.4) did not significantly deteriorate (p=0.068, z=-1.826).
Between admission and discharge, there was a significant decrease in median mMRC grade, indicating reduced dyspnea (p=0.047, z=-1.983). The median mMRC grade between these 2 contacts were respectively 3 (IQR=1) and 2 (IQR=1.5). The BMQ score, divided into “concerns” and “necessity” subcategories, showed a significant decrease in “concerns” at discharge (p=0.011, z=-2.552), while “necessity” remained unchanged (p=0.419, z=-0.808). The median “concerns” score shifted from 11 (IQR=7) to 8 (IQR=4.5) and the median “necessity” score shifted from 18 (IQR=5) to 17 (IQR=4) between both contacts. At follow-up, no significant changes were observed in dyspnea severity (mMRC grade) or the BMQ scores compared to discharge.
The time needed to execute the visits is represented in Figure 1. The first visit was the most labor-intensive (median of 20 minutes, IQR=8.75).
Focus Group Discussion
HCPs’ opinions on the protocol implementation were evaluated during a focus group discussion as shown below.
Theme 1: Multidisciplinary Teamwork for Patients With COPD
Before discussing the new protocol implemented in this study the standard of care before the study was reviewed. Before the study, 2 reference nurses educated COPD patients for 3 hours every 2 weeks.
Up to the time of the study, the execution of individual educational sessions was considered not feasible. Due to variability in daily workload, mostly depending on the presence of staff members, the daily care of the patients was perceived as the main priority. Also, there was generally an insufficient number of nurses. The COVID-19 pandemic also had a significant influence on usual care.
Subtheme 1: Addition of a Clinical Pharmacist to the Multidisciplinary Team
The study was executed by pharmacists only, so the clinical pharmacist was given a prominent role in COPD care. For the future, the participants were very interested in a partnership between nurses and pharmacists, where the nurses can evaluate the educational needs of the specific patient, as they are the experts on daily patient care and, therefore, know the patient. Additionally, all HCPs recognized that (clinical) pharmacists are experts on the pharmacotherapeutic aspects of COPD medication since they have a broad knowledge of adverse events, interactions, and therapy adherence.
Subtheme 2: Defining the Role of the Clinical Pharmacist
Pharmacotherapeutic advice on drug-drug interactions, therapy simplification, and related aspects was regarded as a crucial component of this protocol. It was observed that certain patients had been using the same 2 devices for an extended duration. The period spent in the rehabilitation hospital presented an opportune moment to reassess inhalation medication.
In addition to therapy adjustments, assessing the patient's inhalation capacity in relation to inhalation therapy was considered a very strong aspect of this protocol. Both the nursing and pharmaceutical teams were willing to invest in the In-Check DIAL device28 during educational sessions. Overall, participants expressed optimism regarding a future multidisciplinary collaboration to implement this protocol.
Theme 2: Necessary Adjustments to the Pharmaceutical Protocol for Implementation in Daily Practice
The PHARMACOP protocol was intentionally adapted for this study to fit the specific context and needs. Suggestions were made for further adjustments to the adapted protocol to optimize its implementation and ensure its sustainability in a long-term workflow.
Subtheme 1: Individualization of the Protocol
Through the focus group discussion, it was evident that the participants unanimously supported refining the protocol to better suit the individual needs of each patient. All participants reached a consensus that each patient presents unique requirements.
As per the participants, the initial assessment moment was considered the most important, serving as an opportune occasion to estimate the level of assistance or education required by evaluating the inhalation technique. Based on the inhalation technique scores, the staff thought it efficient to determine the frequency of visits required for the individual patient and/or ascertain the specific information desires.
Subtheme 2: Expanding Patient Education
There were suggestions to involve the patient’s family in the education process, and to also broaden the target population as not only patients with COPD can need education about the inhalation device. Other respiratory indications could also qualify.
Subtheme 3: Supplementary Training
During the discussion, it was also mentioned that the nursing staff required additional training to make a future multidisciplinary protocol possible.
The current lack of training was mostly linked to staff shortages. The COVID pandemic also significantly influenced the nurses’ training. Training that was planned to be repeated after a few years had been canceled. This also meant that new nurses were never trained in inhaler use. The multidisciplinary aspect of the pharmaceutical care protocol was highly applauded because knowledge can be shared through educational moments, increasing interprofessional collaboration in the hospital. Aside from this approach, the nursing staff indicated that they would also prefer organized pharmacist-led training days to broaden their knowledge.
Theme 3: Labor Intensity of the Pharmaceutical Care Protocol
An important part of the protocol implementation is also the practical feasibility including the time needed for HCPs to execute the protocol. After evaluating the results of the time investment (Figure 1), the participants were pleasantly surprised by the time required to perform the different assessments and optimizations.
This convinced them that implementing the care protocol with a future multidisciplinary approach could be achievable. To make the implementation of the protocol even more feasible, integration in the electronic health record (EHR) was considered necessary to facilitate communication between HCPs and document recommendations for changes in inhalation therapy.
Theme 4: Follow-Up After Discharge
The participants recognized the major benefit of comprehensive postdischarge follow-up but also identified practical challenges with follow-up calls. The nursing staff expressed concerns about the feasibility of making these calls, leading to the suggestion that pharmaceutical staff take on this responsibility in the future multidisciplinary approach. Furthermore, a proposal was made to integrate inhalation technique follow-up into ambulatory consultations, as all patients are annually invited to visit the outpatient clinic of the rehabilitation hospital.
Alternatively, a monthly group educational session was also mentioned as an educational option, with the expectation that patients could participate annually.
Discussion
This study highlights the potentially valuable role of a clinical pharmacist in optimizing inhaler use and technique during a rehabilitation hospital stay. As this unique setting has not been previously studied, these findings emphasize the importance of pharmacist-led interventions in improving patient outcomes. Notably, this study is the first to assess the inclusion of a clinical pharmacist in COPD management in a Belgian rehabilitation hospital. During a focus group discussion, a panel of involved HCPs felt that the implementation of a pharmaceutical care protocol for patients with COPD in a rehabilitation hospital would be feasible. The PHARMACOP trial was used as the foundation for the care protocol, as it had previously shown significant benefits to patients with COPD in community pharmacies.15 The inhalation score and medication adherence were significantly higher in the intervention group compared to the control group. Other studies have also highlighted the positive impact of involving community pharmacists in COPD management.29,30 The study by Khdour et al showed that a self-management program led by a clinical pharmacist could add value in improving health outcomes such as quality of life, symptom management, and medication adherence.31 Moreover, the benefit of a clinical pharmacist in an acute hospital setting has been demonstrated extensively in studies evaluating their role on various wards within an acute hospital setting, resulting in, for example, less stress related to therapy, increased clinical efficiency, better pain management, lower utilization of secondary care, and higher patient satisfaction.17-19 Results from research focusing on COPD management in acute hospitals also indicated the added value of the hospital pharmacist, leading to fewer inhalation errors and increased adherence.32-34 The systematic review by Lin et al33 shows growing evidence of hospital pharmacists’ interventions having an impact on adherence and inhalation technique but also health outcomes, economic outcomes, and quality of life. It also addresses the benefit of a multidisciplinary approach and the need for further research.
This study took place in a rehabilitation hospital which offers advantages such as longer patient stays, allowing more time for patient education, and evaluation of inhaler technique.35,36 This means that the educational process can be more intense compared to the acute hospital setting.37,38 The study showed an impact on inhaler therapy appropriateness since the clinical pharmaceutical interventions resulted in a reduction in the number of devices per patient or a switch from device type. The protocol included the integration of tools such as an In-Check DIAL, facilitating the choice of the most optimal inhalation device for the patient. The study also had an impact on inhaler use, resulting in an improved inhalation technique, and resolving almost all major device errors. This improvement was influenced by the pharmacist's intervention as no other HCPs were directly involved. Patient cooperation in this study was very high as only one patient refused to participate, indicating that patients were highly motivated and eager to learn how to use their inhalation therapy correctly. During the focus group discussion, participants acknowledged the added value of involving a clinical pharmacist for education about inhaler therapy. Aside from staff shortages, a lack of training and education about inhalation medication for nursing staff was identified, both of which are aspects that can be resolved by pharmacist participation.39,40 One of the main conclusions of the focus group discussion was that the further adjustment of the protocol with a more multidisciplinary approach would be required, thus significantly benefiting patients with COPD. Additionally, to increase the feasibility of the implementation of the protocol, adjustments were discussed by the participants of the focus group, to achieve a patient-tailored protocol and reduce workload. These adjustments are summarized in Table 3.
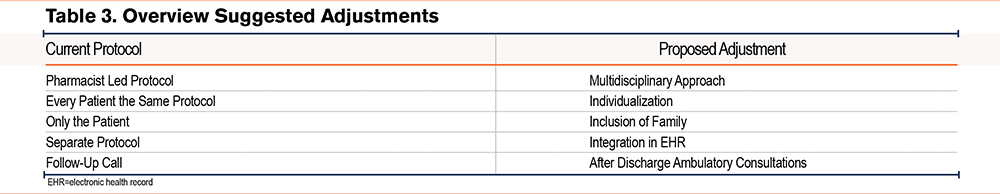
The integration of the protocol in the EHR is essential as this will facilitate interprofessional communication and assist pharmacists during their follow-up of every patient.41-43 As supported by literature, the inclusion of family or other caregivers in educational sessions can positively impact self-management.44 When these caregivers are present at the patient’s home, they can remind them of the important steps and help with medication adherence.
Further adjustments to the protocol were deemed necessary in the discharge and follow-up period (see Appendix 1, part 7 and 8 in the online supplement). A follow-up medication appropriateness screening and evaluation of the inhalation technique can thus be executed. Studies on transmural care showed that early postdischarge patient contact, intensive follow-up of inhalation techniques, and personalized action plans reduce exacerbations.16,45 Another study conducted by Farias et al showed that telehealth systems improve compliance and reduce exacerbations by providing continuous self-management education and easy access to HCPs and prescriptions.46
Additional aspects considering transmural care optimization are also important tools such as a medication scheme and a discharge letter to inform primary HCPs, which we did not include in this study. Secondly, the pharmacist’s follow-up interviews were planned shortly after discharge, and patient knowledge could still be very high. In comparison with a telephone follow-up after 2 weeks, a physical follow-up visit after a longer period could be better. An essential partner in the follow-up is the community pharmacist, who can provide information and evaluate inhaler techniques as well.15 Only 21% of the evaluated patients indicated that they had received an explanation on inhaler use from their community pharmacist. A follow-up consultation with the physician-specialist and nurse in the rehabilitation hospital’s outpatient clinic could also be an appropriate moment to perform such an evaluation.
It would be appropriate to make the required changes and run the protocol within a larger population to establish more evidence on the influence of the adjusted PHARMACOP protocol on patients’ clinical outcomes such as exacerbation rate, inhalation technique, smoking cessation, etc. Although the rehabilitation hospital setting has its advantages, as already discussed, an important limitation is the low patient turnover and, therefore, a low number of patients eligible for inclusion. As a result, an adequately powered cluster-randomized controlled trial in this setting may not be feasible. Based on previous studies, such as the PHARMACOP trial, the addition of a clinical pharmacist in optimization of inhaler therapy can be beneficial and increase quality of care for patients with COPD in different settings, including the rehabilitation hospital. The addition of basic clinical pharmacy services is, however, not financed by the government in rehabilitation hospitals, in contrast to general hospitals in Belgium which plays an important role in importance of workload consideration.47
Conclusion
In conclusion, the implementation of a pharmaceutical care protocol in a rehabilitation hospital was well-received. All involved HCPs were highly motivated to integrate it into their daily practice, considering several adaptations. The patients’ inhalation technique improved significantly during the stay in the rehabilitation hospital after multiple visits involving educational moments, which highlights an important potential role for the clinical pharmacist in the care of these patients.
Acknowledgements
Author contributions: All authors contributed significantly to the conception, design, and execution of the research. ET was responsible for the initial idea and design of the study. AW and EW conducted the data collection and analysis. SCMW provided practical guidance and critical revisions and contributed to the interpretation of the results. KDP, SB, and KS further assisted with the data collection. All authors reviewed and approved the final manuscript.
Declaration of Interests
The authors have no conflicts of interest to declare regarding the publication of this paper.