Running Head: Protocol for the Cluster Randomized PREVAIL Trial
Funding Support: This trial will be conducted by the Observational and Pragmatic Research International Ltd and was co-funded by Observational and Pragmatic Research International and AstraZeneca. No funding was received by the Observational and Pragmatic Research Institute Pte Ltd (OPRI) for its contribution.
Date of Acceptance: April 21, 2025 | Publication Online Date: April 25, 2025
Abbreviations: CONQUEST=COllaboratioN on QUality improvement initiative for achieving Excellence in STandards of COPD care; COPD=chronic obstructive pulmonary disease; CRT=cluster randomized trial; EMR=electronic medical records; ICS=inhaled corticosteroid; IMD=Index of Multiple Deprivation; IRB=institutional review board; ITT=intention to treat; MACRE=major adverse cardiac or respiratory events; NHS=National Health Service; OPCRD=Optimum Patient Care Research Database; OPRI=Observational and Pragmatic Research Institute; PREVAIL=PRagmatic EVAluation of an Improvement program for people Living with modifiable high-risk COPD; PCCs=primary care clusters; QIP=quality improvement program; QS=quality standards; SCS=systemic corticosteroids
Hickman K, Tarabichi Y, Dickens AP, et al. Pragmatic evaluation of an improvement program for people living with modifiable high-risk COPD versus usual care: protocol for the cluster randomized PREVAIL trial. Chronic Obstr Pulm Dis. 2025; 12(3): 223-239. doi: http://doi.org/10.15326/jcopdf.2024.0564
Introduction
The social, economic, and personal burdens resulting from chronic obstructive pulmonary disease (COPD) are well established,1-3 and national strategies such as those in the United States4 and the United Kingdom5have aimed to diagnose COPD earlier, improve management of the disease, and increase awareness among the general population. Despite these campaigns and initiatives and the high prevalence of COPD in high-income countries, reports indicate up to 60% of cases are undetected,6-8 with many patients only receiving a diagnosis after admission to the hospital for an acute exacerbation9 and/or having experienced a significant decline in lung function.10,11 This suggests that opportunities for earlier diagnosis may have been missed, resulting in an increased risk of exacerbations and an associated potential for an increased risk of cardiovascular events, greater lung function decline, higher mortality rates, and increased health care costs.10-18 Three recent cross-sectional database studies in the United Kingdom, United States, and Australia explored clinical management of patients with high-risk COPD, with all studies identifying opportunities for earlier identification of COPD, increased assessment of cardiovascular risk, optimization of inhaled therapy, and referral for appropriate nonpharmacological treatment.19-21
Primary care settings play a pivotal role in COPD care, serving as the first point of contact for patients presenting with symptoms, the primary location for diagnoses, and the central hub for long-term management of the condition. Even if diagnosed, many patients with COPD are at increased risk of future respiratory events and could benefit from improved management, such as regular symptom assessment and counseling on medication adherence, inhaler technique, vaccinations, and smoking cessation.22-31 There is a subgroup of patients, both diagnosed and undiagnosed with potential COPD, who are at greater risk of exacerbations and adverse events (due to a history of previous exacerbations, comorbidities, or clinical characteristics), in whom there may be opportunities to modify that risk through earlier diagnosis and/or improved management and treatment.32-36 We have adopted the concept “modifiable high-risk” to describe this subgroup and introduced a quality improvement program designed to help this population in primary care settings.
The COllaboratioN on a QUality Improvement Initiative to Achieve Excellence in STandards of COPD Care (CONQUEST) program uses routinely collected electronic medical records (EMRs) to identify undiagnosed and diagnosed modifiable high-risk patients and provides a clinical decision support tool for primary care professionals to guide their management, in alignment with expert opinion and national and international guidelines.37 CONQUEST is a quality improvement program (QIP) embedded in primary care that is underpinned by 4 evidence-based quality standards (QSs) that were specifically developed by expert pulmonologists. The QSs integrate quality improvement into routine patient care by supporting health care systems to achieve a timely diagnosis, provide individualized disease assessment, optimize COPD pharmacological and nonpharmacological management, and ensure appropriate follow-up.35 The CONQUEST QIP is novel in that, unlike previous studies focused largely on case-finding,32-34,36,38-40 it combines case-finding with identifying opportunities to optimize management. The scope of the CONQUEST QIP also extends beyond interventions involving case-finding with subsequent treatment,41 by identifying both newly diagnosed and already diagnosed patients at increased risk of future exacerbations due to their exacerbation history,11,42 optimizing their management and monitoring them over a longer period. Previous COPD QIPs have improved guideline adherence, medication prescription, and patients’ inhaler technique.43-45 We anticipate that by identifying patients with modifiable high-risk COPD and focusing on their assessment, treatment, and follow-up, the CONQUEST QIP will improve guideline adherence and patient health outcomes.
As a QIP designed for implementation in primary care settings, it is critical to assess whether the program makes an impact in these settings. The PRagmatic EVAluation of a quality Improvement program for people Living with modifiable high-risk COPD (PREVAIL) pragmatic cluster randomized trials (CRTs), which are described in detail here, have been designed to answer this question. The aim of the PREVAIL trials is to evaluate the health outcomes resulting from CONQUEST QIP implementation in terms of COPD exacerbations and major adverse cardiac or respiratory events (MACREs), in both diagnosed and undiagnosed modifiable high-risk patients, compared to usual care.
Methods
Study Design
The PREVAIL CRTs are 2 separate, but related, cluster randomized pragmatic trials—one conducted in the United Kingdom, the other in the United States—that will test the effectiveness of the CONQUEST QIP in improving patient outcomes and determine its impact compared to usual care among primary care patients who are already diagnosed with COPD, and patients undiagnosed with potential COPD, who meet the modifiable high-risk criteria. Although the trials are independent of each other, they will follow the standardized protocol described in this manuscript.
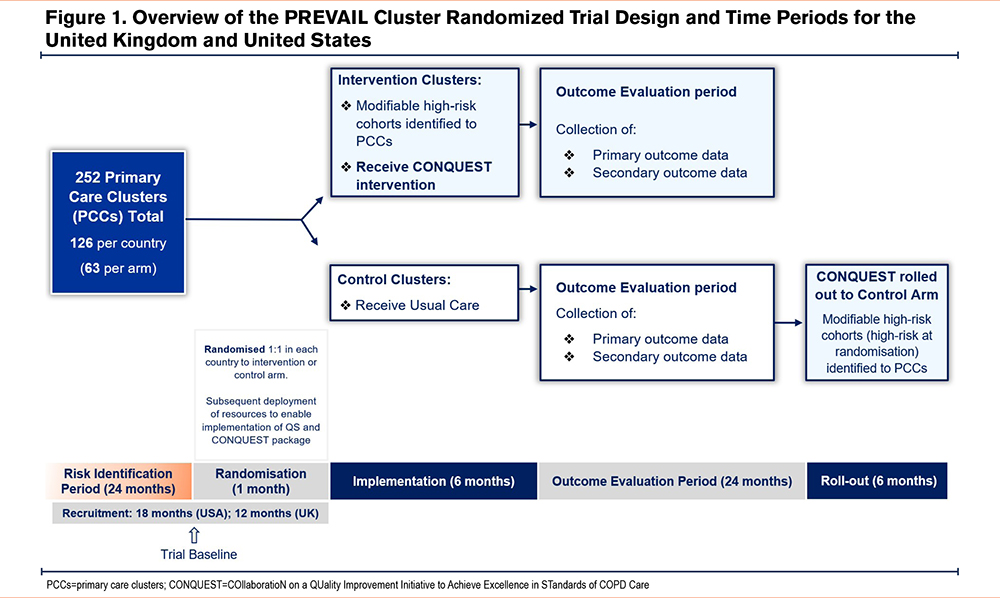
As the CONQUEST QIP is a cluster-level behavioral intervention, the CRT design will avoid the logistical challenges and risk of contamination inherent in equivalent individually randomized trials. Primary care clusters (PCCs) will be the unit of randomization for the trials. PCCs in each country will be randomized 1:1 to either the intervention arm where the CONQUEST QIP will be delivered, or to the control arm where they will continue to deliver usual care until the end of the outcome evaluation period (Figure 1). The CONQUEST QIP will then be rolled out to PCCs in the control arm.
Primary Care Setting
The PREVAIL CRTs will be conducted within primary care settings in order to be generalizable, and more closely aligned with the needs of stakeholders (e.g., patients, practitioners, payers, and institutions involved in public health).46-48 In the United Kingdom, PCCs represent general practitioner practices (primary care organizations serving a list of registered patients), whereas in the United States, self-contained primary care teams (primary care practice personnel identifying as a team caring for a panel of patients49) will form an individual cluster. This variation is required as primary care settings in the United States vary from small independent practices to large clinics within an integrated health system.49
In the United Kingdom, primary care respiratory leads, primary care respiratory networks, and National Health Service (NHS) research organizations will identify PCCs suitable for inclusion. PCCs who have opted to participate in the broader Optimum Patient Care QIP will also be invited to participate in PREVAIL. In the United States, integrated health care systems will identify PCCs within their network that are suitable for inclusion. To be eligible for inclusion in the PREVAIL CRT, PCCs must function as a single randomization unit and have a sufficient number of patients meeting modifiable high-risk criteria, in accordance with sample size calculations. PCCs will be ineligible for inclusion if they are in the process of or planning to change their EMR system within the trial period and/or if they are currently engaged in other COPD-related research studies or QIPs. In both countries, the PREVAIL trial will be conducted in diverse geographical areas to maximize the generalizability of the study findings.
Participant Eligibility Criteria
A total of 3 subsets of modifiable high-risk patients will be included in the PREVAIL CRTs: undiagnosed with potential COPD, newly diagnosed COPD, and already diagnosed COPD. Definitions for patient cohorts and related terms are outlined in Table 1. All patients will be ≥40 years old, have a history of COPD exacerbations (or COPD-like exacerbations if not in the already diagnosed patient group), have ≥24 months of EMR data available, and have a clear opportunity for optimization in disease management. COPD-like exacerbations in undiagnosed patients are identified as events analogous to a COPD exacerbation; episodes of respiratory symptoms treated with courses of antibiotics, steroids, or both. Patients who are undiagnosed but with potential COPD will also have a positive smoking history.
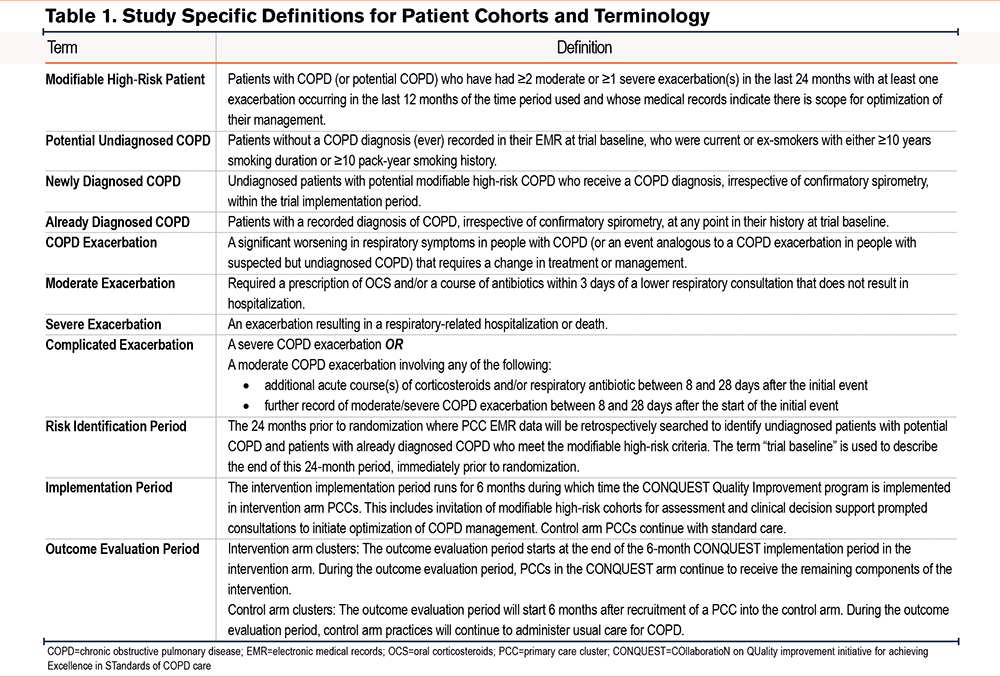
Patients treated with any form of inhaled triple therapy involving long-acting muscarinic antagonists, long-acting beta2-agonists, and inhaled corticosteroids (ICSs), actively treated asthma, and any other significant lung disease and/or active cancer (except for noninvasive skin cancer) will be excluded to ensure that patients receiving the QIP have modifiable disease and are able to participate in the intervention. Patients who do not provide permission to use their EMR data for research will also be excluded.
For PCCs randomized to the intervention arm, modifiable high-risk patients will be identified by applying CONQUEST patient identification algorithms to primary care EMR data covering a 24-month risk identification period. These algorithms are derived from previously validated tools, adapted to meet the specific requirements of the CONQUEST program.38,50-52
Data Source
There will be variations between U.K. and U.S. PREVAIL data repositories because of different ethical and data management requirements. In the United Kingdom, structured and unstructured patient data will be obtained from EMRs using the Optimum Patient Care Research Database (OPCRD).53The OPCRD was established in 2005 and comprises data for over 24 million patients. It has been approved by the U.K. NHS for clinical research use and is validated and frequently utilized for medical and health research.51,54-58
In the United States, the DARTNet Institute will extract standardized, structured EMR data from participating PCCs and provide the Observational and Pragmatic Research Institute (OPRI) with a limited data set. OPRI will house the data in a U.S. cloud-based computing system that is compliant with the Health Insurance Portability and Accountability Act.
Intervention
A detailed description of the CONQUEST QIP has previously been published but in summary, it supports health care professionals in primary care in the identification and management of patients with modifiable high-risk COPD, structured around the 4 CONQUEST QSs.35,37 Figure 2 summarizes the program components, and outlines the activities undertaken and the support given to intervention PCCs during the trial. All considerations for assessment, pharmacological or nonpharmacological management, or follow-up are based on current best practice guidelines and expert opinion at the time of program implementation and are detailed in the CONQUEST Clinical Decision Support package provided to health care providers. The package includes considerations for both pharmacological therapy and nonpharmacological management, such as pulmonary rehabilitation referral, vaccination uptake, smoking cessation advice, and inhaler technique review where applicable.
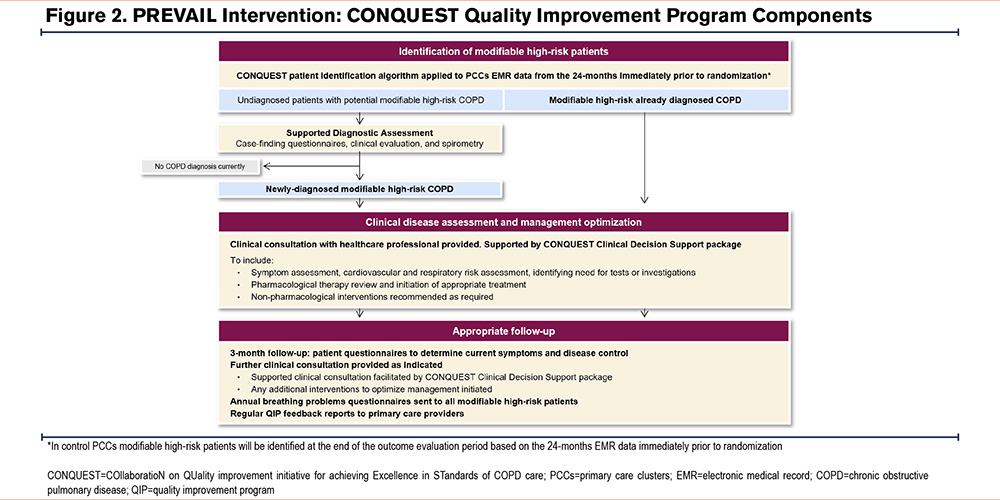
All decisions about patient management are made entirely at the discretion of the treating clinicians and patients, who together negotiate the preferred course of action during consultations in the implementation and outcome evaluation phases of the trial.
Implementation of spirometry testing in undiagnosed patients and the provision of clinical consultations differ between the United Kingdom and the United States based on health care system structure and logistics. For example, the clinical consultation during disease assessment is provided remotely by clinical pharmacists who then discuss treatment considerations with general practitioners in the United Kingdom, and by appointed clinical providers working with primary care practitioners in U.S. PCCs. However, the core components of the intervention are the same.
The implementation of the QIP at PCCs lasts 6 months and during this time the core components of the intervention (except ongoing patient follow-up and QIP feedback reports for PCCs) will be delivered.
Comparator
In the control arm of the PREVAIL CRTs, PCCs will continue to provide usual care until the end of the trial period.
Outcomes
All trial outcomes described below will be assessed over a 24-month period.
Primary Outcomes
The primary outcomes are the annual rate of moderate or severe COPD exacerbations and the annual rate of major adverse cardiac or respiratory events (MACRE). Moderate COPD exacerbations are defined as events requiring oral corticosteroids and/or a course of antibiotics within 3 days of a lower respiratory consultation that does not result in hospitalization; whereas severe COPD exacerbations are defined as events resulting in respiratory-related hospitalization or all-cause mortality.
MACRE extends the composite outcome of major adverse cardiac events, which has been used in many previous studies,59-62 to also include a respiratory component that better reflects the type of adverse events to which patients with modifiable high-risk COPD are exposed. In PREVAIL, MACRE encompasses incident heart failure diagnosis or heart failure hospitalization, nonfatal myocardial infarction, nonfatal ischemic stroke, all-cause mortality, severe COPD exacerbations, and complicated exacerbations (requiring additional hospitalization or corticosteroids/antibiotics between 8–28 days after onset of initial exacerbation). MACRE will be analyzed as a composite measure and also disaggregated by individual components.
Secondary Outcome
The secondary outcome is the annual systemic corticosteroid (SCS) dose used to treat exacerbations. SCS exposure will be measured as the average annual dose, in milligrams, of prednisolone taken via any systemic route. Use of SCS other than prednisolone will be converted to prednisolone equivalent dosing using U.K. or U.S. formulary conversion tables.63-65
Exploratory Outcomes
The first exploratory outcome will be the annual rate of explanatory diagnoses, other than COPD, within the subset of initially undiagnosed patients with potential COPD who do not receive a COPD diagnosis following diagnostic case-finding. Explanatory diagnoses will include incident asthma, chronic heart failure, lung cancer, other chronic respiratory conditions, gastroesophageal reflux disease, and any others identified by the study team via analysis of secondary data. The second exploratory outcome will be the annual rate of unscheduled respiratory care events, defined as any event not constituting a formal COPD review (i.e., respiratory-related emergency department visits and hospitalizations except scheduled office/outpatient visits and respiratory-related primary care visits with no supporting COPD review code or record of dyspnea [modified Medical Research Council dyspnea scale66] or disease-specific health-related quality of life [COPD Assessment Test67] score). The rate of incident pneumonia cases will be explored, in light of potential increased use of ICSs within the intervention arm; other ICS-related adverse events will not be explored as they are outside the scope and resources of the trials. Changes in lung function (spirometry) and disease-specific health-related quality of life (COPD Assessment Test) will also be assessed as exploratory outcomes.
Data Management and Statistical Analysis
Data Management
Data management procedures are standardized across the United Kingdom and the United States. Specifically, all data management procedures will be performed by senior data analysts who will continuously ensure the integrity, validity, and confidentiality of data collection and storage procedures through regular monitoring and periodic assessments of data quality. Data collections are checked for content (data structure and format), completeness (presence of key and optional data files), and continuity. Details of each data extraction and transfer will be logged to an administrative database, and the data made available for processing.
Sample Size Calculation
Sample size estimates for the primary analyses cohort are based on the primary objectives, and all calculations account for the clustered nature of the trial design using appropriate formulas for event rates.68 Estimates are similar for both the U.K. and U.S. studies and are indicative of the potential power to detect trial outcomes using primary care medical record data for patients with high-risk COPD, held in the OPCRD. Specifically, the conservative parameter estimates are based on a database snapshot of modifiable high-risk patients in 2015 from 514 primary care clusters with EMR data.
In each of the U.K. and U.S. trials, it was estimated that enrolling 126 clusters with a mean of 12 modifiable high-risk patients would result in 90% power to detect a 30% reduction in the annual exacerbation rate, equivalent to a rate ratio of 0.7 (and 80% power to detect a 26% reduction [rate ratio of 0.74]). This is based on OPCRD data, with an estimated coefficient of variation between PCCs in this cohort of 0.56 and an annual exacerbation rate of 1.36 over a 24-month follow-up period (α=0.05).
Based on the projected number of clusters, using OPCRD data, it was also estimated there would be 90% power to detect a 32% reduction, equivalent to a rate ratio of 0.68 (and 80% power to detect a 28% reduction [rate ratio of 0.72]) in the annual rate of MACRE events in patients with COPD. This is based on an estimated coefficient of variation between PCCs in this cohort of 0.56 and a control group annual MACRE rate over a subsequent 24-month period of 0.53 (α=0.05).
Randomization and Blinding of Primary Care Clusters
To account for variation in important cluster-level characteristics, a stratified randomization approach will be adopted.
In the United Kingdom, PCCs will be stratified according to deprivation (Index of Multiple Deprivation (IMD)69 score 2019) and scale of opportunity for triple therapy (Quality Outcome Framework COPD list size—number of patients currently prescribed triple therapy). Stratification characteristics were selected based on their prognostic association with the primary outcomes, after confirmation of sufficient variation in national data to ensure different levels of risk between stratified PCCs. Based on OPCRD data, the median IMD score and median number of patients with COPD not prescribed triple therapy will be used as cut-points to create “high risk” and “low risk” groups for both characteristics, creating 4 strata: low/low, low/high, high/high and high/low.
In the United States, PCCs will be stratified based on background exacerbation rates into “high" and “low” groups based on the background COPD exacerbation profile within the PCCs in the preceding 12 months. The cut-off rate for the strata will be the median annual rate of exacerbations.
Within each stratum, PCCs will be randomized at a ratio of 1:1 using permuted blocks of different sizes to receive either the CONQUEST QIP (intervention arm), or to continue to provide usual care (control arm). At the end of the outcome evaluation period, the CONQUEST QIP resources will be implemented in the control arm PCCs.
As the trial is evaluating a cluster-level behavioral intervention, it is not possible to blind the PCCs to the allocation arm. For practical reasons, the operational study team will not be blinded to allocation, but the statistician analyzing the trial outcome data will be blinded.
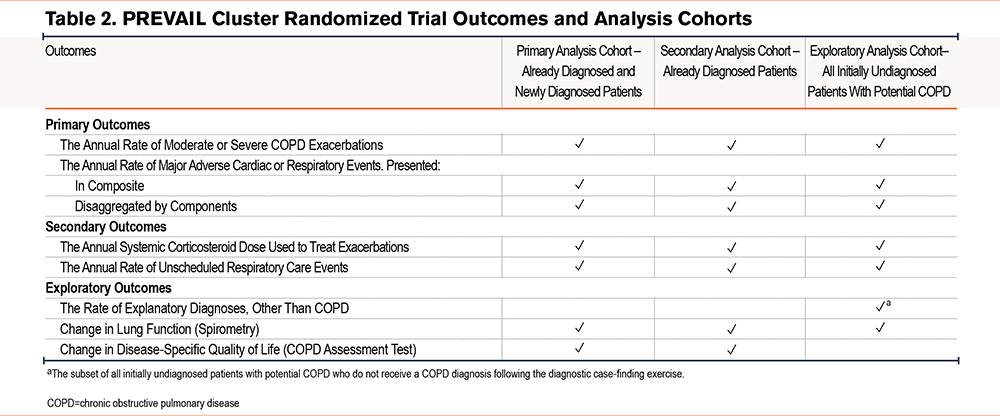
Statistical Analysis
All analyses will be conducted in accordance with a prespecified statistical analysis plan. For the purpose of data analysis, there will be 3 cohorts (Figure 3). The primary analysis cohort will comprise patients with already and newly diagnosed COPD. The secondary analysis cohort will solely comprise patients with already diagnosed COPD, whereas the exploratory analysis cohort will comprise patients who were initially identified as undiagnosed, potential COPD.
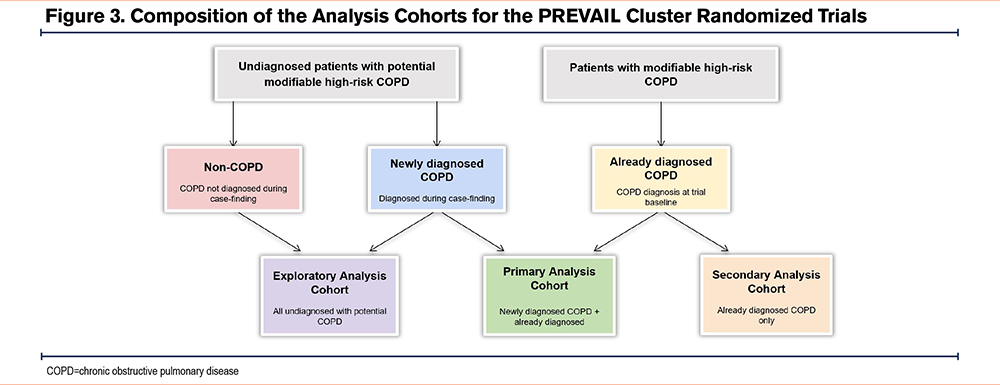
A summary of outcomes crossmatched to analysis cohorts is presented in Table 2, however, all 3 cohorts will be used to examine the primary and secondary outcomes with variations in the cohorts used to assess exploratory outcomes. Data for all primary, secondary, and exploratory outcomes will be analyzed using the intention-to-treat (ITT) principle. To minimize potential bias due to loss to follow-up, any modifiable high-risk patients identified in the control arm who die during the implementation or outcome evaluation period, and who already have a COPD diagnosis code, or where the cause of death is COPD, will be included in the ITT population, even if the patient is not reviewed during the eventual roll-out period.
Poisson regression models with gamma-distributed random effects to account for clustering at the PCC level will be fit to calculate the rate ratio comparing the rates of primary outcomes (dependent variables) between treatment arms (control versus CONQUEST QIP; independent variable), adjusted for main confounders.
All regression models will be adjusted for baseline covariates, such as smoking status, age, socioeconomic status, and sex, as well as the randomization stratification factors of deprivation and the proportion of COPD patients not on triple therapy. Covariates will be prespecified in the Statistical Analysis Plan and included as fixed covariates where appropriate.
An additional per-protocol analysis will assess the impact of the intervention using an independent variable that indicates the level of intervention implementation strength, using a pre-agreed set of indicators that describe the coverage and components of the final CONQUEST QIP. This indicator will be fully developed in parallel with the development of the final intervention package.
Process Evaluation
Data will be collected throughout the QIP implementation phase to determine the extent to which the intervention was delivered as intended, the length and content of assessment clinics, and whether it was delivered to the target population. This process data will provide additional information to inform the interpretation of the trial results and allow judgments on the likelihood of success if the QIP were implemented more widely.
Ethical Approval
In the United Kingdom, the PREVAIL CRT protocol has been approved by the Health Research Authority and the East Midlands – Derby Research Ethics Committee (REC 21/EM/0252) and by the Anonymized Data Ethics and Protocol Transparency Committee (ADEPT 1321). The trial will be performed in compliance with Good Clinical Practice and Good Pharmacoepidemiology Practice and is registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP/DSPP/42512) and with the ISRCTN registry (ISRCTN15819828). In the United States, BRANY Institutional Review Board (IRB) serves as the single IRB (22-08-112) and the trial has been registered with the U.S. National Institutes of Health’s National Library of Medicine’s international Clinical Trials database at (#NCT05306743).
Discussion
The aim of the PREVAIL CRTs is to evaluate the effectiveness of the CONQUEST QIP which focuses on identifying undiagnosed and diagnosed patients with modifiable-high risk COPD and, once identified, improving COPD care in line with expert opinion and evidence-based guidelines. Prior investigations suggest that while case-finding methods can suitably identify patients with COPD, poor follow-up and suboptimal management may prevent improved patient outcomes.23,32-34,38,39,70,71 In this regard, the CONQUEST QIP is novel and has the potential to address these evidence-practice gaps because it combines case-finding with clinical decision tools to guide management. Additionally, the CONQUEST QIP has the potential to meet COPD research priorities identified by the International Primary Care Respiratory Group, such as having simple tools that enable the diagnosis and assessment of COPD in primary care settings, including those with limited access to resources, and managing patients who have COPD and comorbid conditions, including cardiovascular disease.72,73 While ambitious in their proposed undertaking, the PREVAIL CRTs provide an opportunity to compare a wide range of health outcomes in patients with COPD being treated in primary care over a comparatively long time frame.
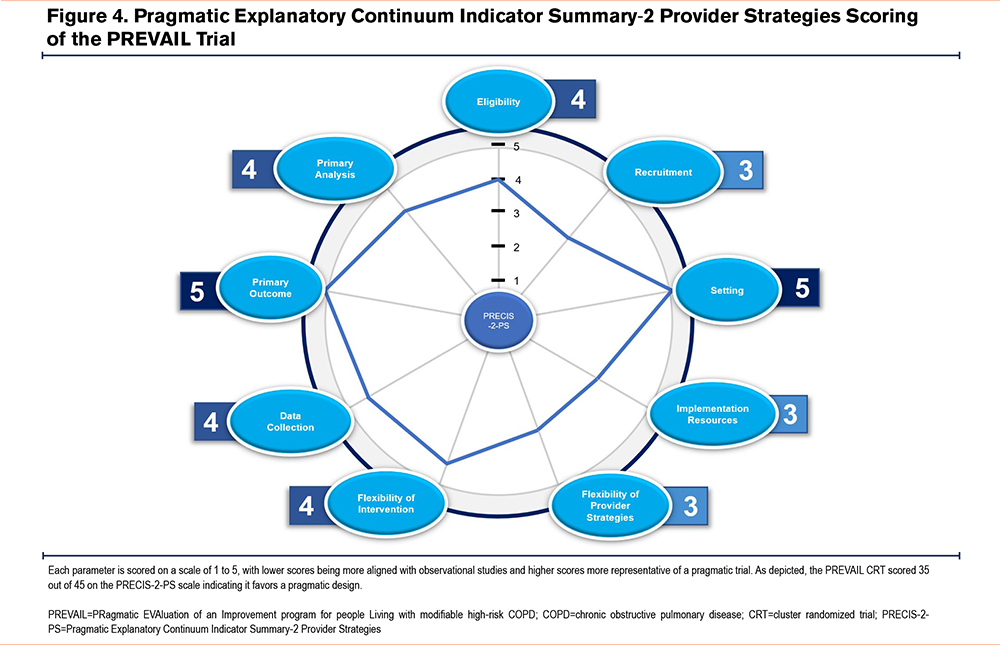
It is important to ensure the PREVAIL CRTs are pragmatically designed, and the methodology is appropriate for assessing the stated outcomes. The PREVAIL CRTs scored 35 out of 45 on the Pragmatic Explanatory Continuum Indicator Summary-2 Provider Strategies tool74; a tool used to identify where planned studies are on the pragmatic-explanatory continuum, with scores ranging from 9 (very explanatory) to 45 (very pragmatic) (Figure 4). Having a pragmatic study design ensures the proposed studies will examine the benefits of the CONQUEST QIP in real-world primary care practice.
The CRT design is appropriate to evaluate the CONQUEST QIP as it is implemented at the PCC-level and aims to change care pathways for patients with modifiable high-risk COPD. CRTs are widely used to evaluate QIPs within health care settings, whereby the health care provider, rather than the individual patient, is the unit of randomization.75-78 The international nature of the PREVAIL CRT will aid assessment of the external validity of the CONQUEST QIP, and the randomization of PCCs will enable the trials to assess the applicability of the QIP and clinical decision support tools to a diverse population of patients with modifiable high-risk COPD living in high-income countries.
Having a 24-month lead-in time prior to baseline randomization will help detect respiratory events in patients with undiagnosed potential COPD who have an increasing frequency of events prior to diagnosis. For example, in a U.S. study,79 it was shown that in the 24 months prior to diagnosis, the mean number of exacerbations for patients with COPD was 2.04. A 24-month risk identification period will also better account for seasonal effects and potential temporary changes in detection, coding, and management of COPD exacerbations due to the COVID-19 pandemic. The follow-up period of at least 24 months will also allow for these factors and give a better estimate of the effects of the program on the risk of exacerbations and MACRE compared to the more common 52 weeks used by many clinical trials.
COVID-19 has had a global impact on clinical trials and research across diverse medical fields, with delays in participant enrollment, operational gaps in conducting trials, and overall delays in projected timelines.80 It is reasonable to assume these challenges could also be experienced in the PREVAIL CRTs, and the additional difficulties of conducting a respiratory-based study in the COVID-19 era must be considered.81 Respiratory-related considerations pertaining to the PREVAIL CRTs include the impact of COVID-19 on COPD exacerbation frequency in the risk identification period, ability and willingness of patients to attend spirometry, and the capacity of participating PCCs to commit to a QIP. The numbers of patients meeting the eligibility criteria will be monitored by the internal study team, and if fewer than anticipated modifiable high-risk patients are identified due to lower baseline exacerbation rates than expected, protocol modifications will be discussed with the study team.
One of the criteria used to identify undiagnosed patients with potential COPD was a positive smoking history; while this maximized the potential yield of newly diagnosed COPD, the case-finding process would have missed undiagnosed COPD within nonsmoking patients. As the trials target patients with the largest scope for optimization in terms of both pharmacological and nonpharmacological management, some patients may be omitted who require appropriate management relating to one of these components. The trial analysis will be based on EMR data in the United Kingdom and the United States, with algorithms extracting data from multiple clinical systems in each country. Use of a common data model82 to standardize the structure and content of extracted EMR data will ensure that data in different formats can be processed and analyzed within the 2 countries; however, variation in clinicians’ recording and coding of patient data means that outcomes may be under- or overestimated. While within-country data variations will be addressed using the above approach, differences in the availability of recorded data in the United Kingdom and the United States precluded analysis of combined data from both countries.
The environmental impact of COPD inhaled therapy is a major concern for health care providers in the United Kingdom and the United States; metered-dose inhalers accounted for approximately 3% of the NHS carbon footprint83 in 2019, and 98% of all inhaler-related emissions in the United States84 in 2022. As the PREVAIL trials have been designed to explore patient health outcomes associated with the quality improvement program, the trials do not include environmental outcomes. However, the licensing of new propellants is likely to change the carbon cost of inhalers considerably during the outcome evaluation period of the trials, rendering interpretation of any collected data during this period challenging.
If the CONQUEST QIP is shown to be effective in improving outcomes for patients with modifiable high-risk COPD, a post hoc economic evaluation may be undertaken to explore the cost-effectiveness of the intervention. Furthermore, the logistics of implementing this program in routine clinical practice will be explored by actively seeking feedback from health care professionals in the intervention arm PCCs and, in collaboration with steering committee members, considering ways of mitigating obstacles in the future.
Should the trials demonstrate successful implementation of the CONQUEST QIP in primary care and beneficial patient health outcomes, they will help to establish the role of automated modifiable high-risk patient identification and the value of optimizing therapy in this patient population. It will also provide a framework for COPD case-finding and quality improvement that can address opportunities for management in this common but often underrecognized and undertreated disease.
Acknowledgements
Author contributions: The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors made a significant contribution to the work reported, whether that is in the study conception, design, or execution; acquisition of data, analysis, and interpretation, or in all these areas. All authors took part in drafting, revising, or critically reviewing the article. All authors gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work. All authors have given approval for the submission of this article. The authors received no direct compensation related to the development of the manuscript.
Other acknowledgments: We would like to acknowledge Shay Soremekun of the Observational and Pragmatic Research Institute, Singapore, and David Jones of Optimum Patient Care, Cambridge, United Kingdom for intellectual contribution to the trial design which supported the development of this publication. Writing, editorial support, and/or formatting assistance in the development of this manuscript was provided by Shilpa Suresh (MSc) of the Observational and Pragmatic Research Institute, Singapore.
Declaration of Interests
KH, YT, MG, CM, GD, MM, and DW report no conflicts of interest.
APD, RP, and AE are employees of the Observational and Pragmatic Research Institute, which is a research collaborator of the CONQUEST initiative with Optimum Patient Care and AstraZeneca.
MK and AC are contracted by the Observational and Pragmatic Research Institute, a research collaborator for the CONQUEST initiative, and by Optimum Patient Care Global, a co-funder of the CONQUEST initiative.
JG and VC are employees of Optimum Patient Care Ltd, a co-funder of the CONQUEST initiative.
LA has served as an advisor or consultant for AstraZeneca, GlaxoSmithKline, and Merck Sharp & Dohme; served as a speaker or a member of a speakers’ bureau for AstraZeneca, GlaxoSmithKline, BIAL, Viatris, and Novartis Pharmaceuticals Corporation.
CA was an employee of the Observational and Pragmatic Research Institute, which is a research collaborator of the CONQUEST initiative with Optimum Patient Care and AstraZeneca.
MB reports grants from AstraZeneca and personal fees and nonfinancial support from AstraZeneca, Chiesi, GlaxoSmithKline, and others from AlbusHealth, outside the submitted work.
JC has received research grants or consultancy fees from Astrazeneca, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, and Zambon.
RC has received grant support from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline; and reimbursement for advisory work and educational activities from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis.
MBD has received grant support from the National Institutes of Health (NIH), Boehringer-Ingelheim, Midmark, and Teva; consultancy fees from AstraZeneca, GlaxoSmithKline, Genentech, Stratos, Verona, and Takeda in the prior 18 months unrelated to this work.
DMGH has received sponsorship to attend international meetings, and honoraria for lecturing, attending advisory boards, and preparing educational materials from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Pfizer.
NAH served as an advisor or consultant for AstraZeneca, GlaxoSmithKline, Sanofi, Genentech, Teva, Verona, and Amgen, and his institution received research grant support from AstraZeneca, GlaxoSmithKline, Sanofi, Genentech, and Teva.
JRH has received personal payment and payment to his institution (UCL), including research grants, reimbursement for advisory work, and educational activities, and support to attend meetings from pharmaceutical companies that make medicines to treat COPD, which include AstraZeneca, Boehringer Ingelheim, Chiesi, and Novartis.
KK has received honoraria for presentations and/or consultancy fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, ELPEN, GSK, Guidotti, Menarini, Pfizer, Sanofi, and Specialty Therapeutics. His department has received funding and/or grants from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GSK, Menarini. He worked with AstraZeneca as Global Medical Head Respiratory Biologics – 02.09.2024 to 29.11.2024. He is a member of the GOLD Assembly.
BM reports funding from the NHLBI for the COPDGene study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovian; personal fees for data safety monitoring boards from Spiration and Shire/Baxalta; CME personal fees from WebMD, National Jewish Health, the American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications Medscape, Eastern VA Medical Center, Academy Continued Health Care Learning, and Mt. Sinai Medical Center; royalties from Up-To-Date; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Vernoa; and grants from Pearl, outside the submitted work.
DM has received grant funding from Boehringer Ingelheim Pharmaceuticals, GlaxoSmithKline, and Sunovion; and consultancy fees from Boehringer Ingelheim Pharmaceuticals, Mylan, Theravance Biopharma, and Novartis.
JM, TM, HM, MP, and FT are employees of AstraZeneca and hold stock and/or stock options in the company. AstraZeneca is a co-funder of the CONQUEST initiative.
FM has received personal fees and nonfinancial support from the American College of Chest Physicians, AstraZeneca, Boehringer Ingelheim, ConCert, Genentech, GlaxoSmithKline, Inova Fairfax Health System, Miller Communications, the National Society for Continuing Education, Novartis, Pearl Pharmaceuticals, PeerView Communications, Prime Communications, Puerto Rico Respiratory Society, Chiesi, Sunovion, Theravance, Potomac, the University of Alabama Birmingham, Physicians Education Resource, Canadian Respiratory Network, Teva, and Dartmouth; nonfinancial support from ProterrixBio, Gilead, Nitto, and Zambon; and personal fees from Columbia University, Integritas, MD magazine, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape, Western Connecticut Health Network, Patara/Respivant, PlatformIQ, the American Thoracic Society, Rockpointe, Rare Disease Health Care Communications, and France Foundation; grant support from the NIH; and is a member of steering committees for Afferent/Merck, Biogen, Veracyte, Prometic, Bayer, Bridge Biotherapeutics, and ProMedior.
MM reports speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols, and Novartis; consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, Palobiofarma SL, pH Pharma, Novartis, Sanofi, and Grifols and research grants from Grifols.
RM is contracted by the Observational and Pragmatic Research Institute, a research collaborator for the CONQUEST initiative, and by Optimum Patient Care Global, a cofunder of the CONQUEST initiative.
SM reported lecture and advisory fees from Novartis Pharma, GlaxoSmithKline, and AstraZeneca; lecture fees and grants from Boehringer Ingelheim; research grants from ROHTO Pharmaceutical and Kintetsu Cable Network, and grants and funding from Chugai Pharmaceutical, Ono Pharmaceutical, and Taiho Pharmaceutical.
CN is an employee of AstraZeneca and holds stock and/or stock options in the company. AstraZeneca is a cofunder of the CONQUEST initiative.
JO has participated in advisory boards for Sunovion Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Mylan, Verona, and Theravance and has received grant funding from Sunovion Pharmaceuticals Inc, TEVA, the Gordon and Betty Moore Foundation, an NIH subaward, and Boehringer Ingelheim.
WP has received funding via subcontracts with his organization from the CDC, PCORI, the NIH, Boehringer Ingelheim, ONC, Tabula Rasa Healthcare, and Astra-Zeneca. His organization received consulting fees for his work from Boehringer Ingelheim. His organization has received payment for expert testimony. He owns stock through a trust in Johnson and Johnson, Eli Lily, Novo-Nordisk, Pfizer, Novartis, Moderna, and Amgen. He received grant and writing support for an unrelated project from Boehringer Ingelheim and was an unpaid member/chair of the Colorado Medicaid Provider Rate Review Committee.
JQ has been supported by institutional research grants from the Medical Research Council, National Institute of Health and Care Research, Health Data Research, GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Insmed, and Sanofi and received personal fees for advisory board participation, consultancy, or speaking fees from GlaxoSmithKline, Boehringer Ingelheim, Sanofi, Chiesi, and AstraZeneca.
AS serves on advisory boards for diabetes, heart failure and osteoporosis for Novartis, Merck Sharp & Dohme, and Boehringer Ingelheim, Eli Lilly, and Amgen.
MKH reports personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, Amgen, UpToDate, Altesa Biopharma, Medscape, NACE, MDBriefcase, Integrity, and Medwiz. She has received either in-kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, Astrazeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. She has participated in data safety monitoring boards for Novartis and Medtronic with funds paid to the institution. She has received stock options from Meissa Vaccines and Altesa Biopharma.
AK is a member of the advisory board of, or speakers bureau for, ALK, AstraZeneca, Belus, Boehringer Ingelheim, Covis, Eisai, GlaxoSmithKline, Idorsia, Merck Frosst, Moderna, Novo Nordisk, Novartis, Pfizer, Purdue, Sanofi, Teva, Trudel, and Valeo.
DS has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, EpiEndo, Genentech, GlaxoSmithKline, Glenmark, Gossamer Bio, Kinaset Therapeutics, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Teva, Theravance Biopharma, and Verona Pharma. He is supported by the National Institute for Health Research Manchester Biomedical Research Centre.
MS reports previously received fees from AstraZeneca, Boehringer Ingelheim, Cheisi, GlaxoSmithKline, Napp/Mundipharma, Pfizer, and Teva.
T. Wilkinson has received personal fees from AstraZeneca, GlaxoSmithKline, Enanta, Epiendo, Johnson & Johnson, Novartis, Genentech, Synairgen, my mhealth, bioMerieux, Roche and Sanofi/Regeneron alliance. Research grants from AstraZeneca, bioMerieux, GlaxoSmithKline, my mhealth, synairgen, Janssen, and Sanofi/Regeneron.
T. Winders has received personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Genentech, and Sanofi/Regeneron alliance. The Allergy & Asthma Network and the Global Allergy & Airways Patient Platform have received funds for unbranded disease awareness and education from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Genentech, Viatris, and Sanofi/Regeneron.
DP has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, and Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, and Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and the U.K. National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, and Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, and Teva Pharmaceuticals. He owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and U.K.) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore). He reports 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the U.K. Efficacy and Mechanism Evaluation programme, and Health Technology Assessment and was an expert witness for GlaxoSmithKline.